

色胺酮衍生物设计、合成及抗肿瘤活性构效关系研究
收稿日期: 2015-07-15
修回日期: 2015-09-14
网络出版日期: 2015-09-25
基金资助
陕西省自然科学基金(No. 2014JM4095)、陕西省教育厅基金(No. 12JK1010)和陕西省重点科技创新团队计划(No. 2013KCT-24)资助项目.
Design, Synthesis and Structure-Activity Relationship ofTryptanthrins as Antitumor Agents
Received date: 2015-07-15
Revised date: 2015-09-14
Online published: 2015-09-25
Supported by
Project supported by the Shaanxi Provincial Natural Science Fundation (No. 2014JM4095), the Foundation of the Education Department of Shaanxi Province (No. 12JK1010), the Key Program for Science and Technology Innovative Research Team of Shaanxi Province (No. 2013KCT-24).
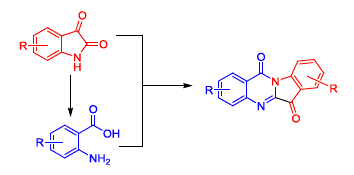
首先合成吲哚醌衍生4a~4f, 氧化水解得到邻氨基苯甲酸衍生物5a~5d. 以这两者为原料设计合成A和/或D环取代的色胺酮衍生物1a~1q. 然后, 以色胺酮6位酮羰基分别与水合肼、盐酸羟胺反应生成C环席夫碱结构. 最后, 以哌嗪结构取代B环嘧啶酮合成茚(1,2-b)喹喔啉-11-酮. 共设计合成20个化合物, 其中新化合物13个. 对所合成化合物的结构经红外光谱、核磁氢谱、元素分析确证. 测定所合成化合物对肿瘤细胞A549的体外抑制活性. 结果表明化合物1b, 1c, 1i, 1j, 1p和1q表现出较强的肿瘤细胞抑制活性, IC50值分别为3.58, 0.99, 1.03, 2.10, 0.51和0.43 μmol·L-1. 构效关系研究表明: D环卤素取代提高抗肿瘤活性, 而取代基团在A环时则减弱抗肿瘤活性; B环(嘧啶环)被哌嗪环取代后抗肿瘤活性消失(IC50 >100 μmol·L-1); 而C环酮羰基生成席夫碱结构抗肿瘤活性与色胺酮相当.
侯宝龙 , 艾芸 , 王翠玲 , 张宁 , 杨柳 , 刘竹兰 , 刘建利 . 色胺酮衍生物设计、合成及抗肿瘤活性构效关系研究[J]. 有机化学, 2016 , 36(1) : 121 -129 . DOI: 10.6023/cjoc201507012
The isatin derivatives 4a~4f were prepared and underwent oxidative hydrolysis to give the anthranilic acid 5a~5d. A and/or D-ring substituted tryptanthrins were designed and synthesized from 4a~4f to 5a~5d. Then C-ring Schiff bases of tryptanthrin were synthesized by condensation of 6-carbonyl with hydrazine and hydroxylamine hydrochloride. Finally, the B-ring was replaced with piperazine to give 11H-indeno[1,2-b]quinoxalin-11-one. 20 compounds were synthesized and their structures were confirmed by 1H NMR, IR and elemental analysis. To best of our knowledge, 13 of them were unknown in the literature. The antitumor activities of synthesized compounds were evaluated against A549 cell line in vitro. The preliminary results indicated that 1b, 1c, 1i, 1j, 1p and 1q showed good antitumor activity with the IC50 of 3.58, 0.99, 1.03, 2.10, 0.51 and 0.43 μmol·L-1, respectively. Structure-activity relationship showed that halogen substitution located in the D-ring enhanced the anti-tumor activity, while the same substitution located in the A ring reduced the activity. The anti-tumor activity disappeared when B-ring was replaced by piperazine, while there was no significant difference for tryptanthrin and its C-ring Schiff base.

Key words: tryptanthrin; isatin; anthranilic acid; antitumor activity
[1] Honda, G.; Tabata, M. Planta Med. 1979, 36, 85.
[2] Honda, G.; Tosirisuk, V.; Tabata, M. Planta Med. 1980, 38, 275.
[3] Henning, D.; Dietmar, B.; Matthias, H. Planta Med. 2002, 68, 152.
[4] Schindler, W.; Zähner, H. Arch. Mikrobiol1971, 79, 187.
[5] Yurngdong, J. Arch. Pharm. Res. 2013, 36, 517.
[6] Bandekar, P. P.; Roopnarine, K. A.; Parekh, V. J.; Mitchell, T. R.; Novak, M. J.; Sinden, R. R. J. Med. Chem. 2010, 53, 3558.
[7] Takel, Y.; Kunikata, T.; Aga, M.; Inoue, S.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. J. Biol. Pharm. Bull. 2003, 26, 365.
[8] Taterao, M.; Sachin, P.; Kumar, A.; Srinivasan, V. I. ARKIVOC 2008, xiv, 100.
[9] Bhattacharjee, A. K.; Skanchy, D. J.; Jennings, B.; Hudson, T. H.; Brendle, J. J.; Werbovetz, K. A. Bioorg. Med. Chem. 2002, 10, 1979.
[10] Sharma, V. M.; Prasanna, P.; Seshu, K. V. A.; Renuka, B.; Rao, C. V. L.; Kumar, G. S.; Narasimhulu, C. P.; Babu, P. A.; Puranik, R. C.; Subramanyam, D.; Venkateswarlu, A.; Rajagopal, S.; Kumar, K. B. S.; Rao, C. S.; Mamidi, N. V. S. R.; Deevi, D. S.; Ajaykumar, R.; Rajagopalan, R. Bioorg. Med. Chem. Lett. 2002, 12(17), 2303.
[11] Yu, S. T.; Chern, J. W.; Chen, T. M.; Chiu, Y. F.; Chen, H. T.; Chen, Y. H. Acta Pharmacol. Sin. 2010, 31, 259.
[12] Pathnia, A. S.; Kumar, S.; Guru, S. K.; Bhushan, S.; Sharma, P. R.; Aithagani, S. K.; Singh, P. P.; Vishwakarma, R. A.; Kumar, A.; Malik, F. PLoS One 2014, 9, e110411.
[13] Hwang, J. M.; Oh, T.; Kaneko, T.; Upton, A. M.; Franzblau, S. G.; Ma, Z.; Cho, S. N.; Kim, P. J. Nat. Prod. 2013, 76, 354.
[14] Pergola, C.; Jazzar, B.; Rossi, A.; Northoff, H.; Hamburger, M.; Sautebin, L.; Werz, O. Br. J. Pharmacol. 2012, 165, 765.
[15] Friedlander, N.; Roschdestwensky, P. Chem. Ber. 1915, 48, 1841.
[16] Eguehi, S. ARKIVOC 2005, 11, 98.
[17] Bergman, J.; Lindstrm, J. O.; Tilstam, U. Tetrahedron 1985, 41, 2879.
[18] Yu, S. T.; Chern, J. W.; Chen, T. M.; Chiu, Y. F.; Chen, H. T.; Chen, Y. H. Acta Pharmacol. Sin. 2010, 31, 259.
[19] Wang, C. L.; Hou, B. L.; Zhang, N.; Sun, Y. N.; Liu, J. L. Chem. J. Chin. Univ.2015, 36, 274 (in Chinese).(王翠玲, 侯宝龙, 张宁, 孙艳妮, 刘建利, 高等学校化学学报, 2015, 36, 274.)
[20] Wang, Z.; Wang, C. L.; Sun, Y. N.; Zhang, N.; Liu, Z. L.; Liu, J. L. Tetrahedron 2014, 70.
[21] Gao, W. T.; Zhao, P. B.; Zhao, B. B.; Li, Y. Chin. J. Org. Chem. 2014, 34, 126 (in Chinese).(高文涛, 赵鹏波, 赵宾宾, 李阳, 有机化学, 2014, 34, 126.)
[22] Wang, C. L.; Liu, Z. L.; Hou, B. L.; Wang, J.; Zhang, N.; Liu, J. L. J. Northwest Univ.(Nat. Sci. Ed.) 2011, 41, 817 (in Chinese).(王翠玲, 刘竹兰, 侯宝龙, 王瑾, 张宁, 刘建利, 西北大学学报(自然科学版), 2011, 41, 817.)
[23] Stiff, C.; Graber, D. R.; Thorarensen, A.; Wakefield, B. D.; Marotti, K. R.; Melchior, E. P.; Sweeney, M. T.; Han, F.; Rohrer, D. C.; Zurenkoc, G. E.; Romeroa, D. L. Bioorg. Med. Chem. Lett. 2008, 18, 6293.
[24] Zhao, Y.; Ouyang, G. P.; Xu, W.M.; Jin, L.H.; Yuan, K. Chin. J. Org. Chem. 2010, 30, 1093 (in Chinese).(赵云, 欧阳贵平, 徐维民, 金林红, 袁凯, 有机化学, 2010, 30, 1093.)
[25] Zhou, W.; Liu, X. F.; Tu, Z. C.; Zhang, L. W.; K, X.; Bai, F.; Zhao, Z. J.; X, Y. F.; Deng, K.; Li, H. L. J. Med. Chem. 2013, 56, 7821.
[26] Wu, C. H.; Coumar, M. S.; Chu, C. Y.; Lin, W. H.; Chen, Y. R.; Chen, C. T.; Shiao, H. Y.; Rafi, S.; Wang, S. Y.; Hsu, H.; Chen, C. H.; Chang, C. Y.; Chang, T. Y.; Lien, T. W.; Fang, M. Y.; Yeh, K. C.; Chen, C. P.; Yeh, T. K.; Hsieh, S. H.; Hsu, J. T. A.; Liao, C. C.; Chao, Y. S.; Hsieh, H. P. J. Med. Chem. 2010, 53, 7316.
[27] Chen, Y.; Bai, S.; He, H. W.; Yang, G. Z. Chin. J. Org. Chem. 2014, 34, 2362 (in Chinese).(陈玉, 柏舜, 贺红武, 杨光忠, 有机化学, 2014, 34, 2362.)
[28] Zhou, L. H.; Tu, S. J.; Shi, D. Q. J. Chem. Res. 1998, 398.
[29] Andreas, G.; Guenther, S.; Gerhard, W. DE 4114990, 1992 [Chem. Abstr. 1993, 119, 10457].
[30] Bogdana, K.; Amber, C. N.; Kelsi, A. D.; Peter, G. Bioorg. Med. Chem. Lett. 2013, 23, 1032.
[31] Manickam, B.; Raman, S.; Jayakumar, S. Tetrahedron Lett. 2014, 55, 5808.
/
| 〈 |
|
〉 |