

微波促进下含苯并杂环芳硫醚的合成
收稿日期: 2015-09-21
修回日期: 2015-10-14
网络出版日期: 2015-10-26
基金资助
山西省科技创新项目(No. 2014101011)及山西大学本科生科研训练(No. 2014013)资助项目
Synthesis of Heterocyclic Aromatic Sulfides under Microwave Irradiation
Received date: 2015-09-21
Revised date: 2015-10-14
Online published: 2015-10-26
Supported by
Project supported by the Science and Technology Innovation Project of Shanxi Province (No. 2014101011) and the Undergraduate Research Training Program of Shanxi University (No. 2014013).
张变香 , 杨丽花 , 史瑞雪 , 亢永强 . 微波促进下含苯并杂环芳硫醚的合成[J]. 有机化学, 2016 , 36(2) : 352 -357 . DOI: 10.6023/cjoc201509026
The reaction of the thiolazole compounds with aryl iodide resulted in a series of aryl thioether compounds under microwave irradiation. It showed that the yield of target product was 88% when the reaction was carried out under microwave (30 W) heating for 15 min in N,N-dimethylformamide (DMF). The target products using microwave response and oil bath heating were tracked separately by high performance liquid chromatography (HPLC) analysis under the same conditions. The results showed that the method of microwave irradiation for the preparation of aryl sulfide was simple, efficient, time-saving and less by-products. It is expected to become an efficient, gentle and environmentally friendly synthetic method of heterocyclic aryl sulfides.
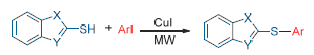
[1] Xiao, F.; Chen, H.; Xie, H.; Chen, S.; Yang, L.; Deng, G. Org. Lett. 2014, 16, 50.
[2] Inomata, H.; Toh, A.; Mitsui, T.; Fukuzawa, S. Tetrahedron Lett. 2013, 54, 4729.
[3] Varun, B.; Prabhu, K. J. Org. Chem. 2014, 79, 9655.
[4] Alves, D.; Lara, R.; Contreira, M.; Radatz, C.; Duarte, L.; Perin, G. Tetrahedron Lett. 2012, 53, 3364.
[5] (a) Azam, M.; Suresh, B. Sci. Pharm. 2012, 80, 789. (b) Pejin, B.; Iodice, C.; Tommonaro, G. J. Nat. Prod. 2008, 71, 1850. (c) Wei, H.; Yang, G. Bioorg. Med. Chem. 2006, 14, 8280. (d) Gangjee, A.; Zeng, Y.; Talreja, T. J. Med. Chem. 2007, 50, 3046.
[6] Xiao, S.; Zhu, J.; Mu, X. Chin. J. Org. Chem. 2013, 33, 1668 (in Chinese). (肖尚友, 朱俊, 穆小静, 有机化学, 2013, 33, 1668.)
[7] Qiao, Z. J.; Liu, H.; Xiao, X.; Fu, X. Org. Lett. 2013, 15, 2594.
[8] Wang, B.; Graskemper, J. W.; Qin, L.; DiMagno, S. Angew. Chem., Int. Engl. 2010, 49, 4079.
[9] Liu, K.; Ou, H.; Shi, X. J. Org. Chem. 2014, 4, 681.
[10] Kumat, S.; Engman, L. J. Org. Chem. 2006, 71, 5400.
[11] Wang, D.; Yu, X.; Zhao, K.; Li, L.; Ding, Y. Tetrahedron Lett. 2014, 55, 5739.
[12] Taniguchi, N. J. Org. Chem. 2006, 71, 7874.
[13] Ge, W.; Wei, Y. Green Chem. 2012, 14, 2066.
[14] Luo, P.; Yu, M.; Tang, R.; Zhong, P.; Li, J. Tetrahedron Lett. 2009, 50, 1066.
[15] Sekar, R.; Srinivasan, M.; Marcelis, A.; Sambandam, A. Tetrahedron Lett. 2011, 52, 3347.
[16] He, Z.; Luo, F.; Li, Y.; Zhu, G. Tetrahedron Lett. 2013, 54, 5907.
[17] Mondal, J.; Borah, P.; Modak, A.; Zhao, Y. L.; Bhaumik, A. Org. Process Res. Dev. 2014, 18, 257.
[18] Zhang, X.; Zeng, W.; Yang, Y.; Huang, H.; Liang, Y. Org. Lett. 2014, 16, 876.
[19] Song, H.; Leninger, M.; Lee, N.; Liu, P. H. Org. Lett. 2013, 15, 4854.
[20] Varala, R.; Ramu, E.; Alam, M.; Adapa, S. Chem. Lett. 2004, 33, 1614.
[21] Jalalian, N.; Petersen, T.; Olofssn, B. Chem. Eur. J. 2012, 18, 14140.
[22] He, G.; Huang, Y.; Tong, Y.; Zhang, J.; Zhao, D.; Zhou, S.; Han, S. Tetrahedron Lett. 2013, 54, 5318.
[23] Zhang, B.; Chen, K.; Yang, L. Chin. J. Org. Chem. 2015, 35, 905 (in Chinese). (张变香, 陈凯, 杨丽花, 有机化学, 2015, 35, 905.)
[24] Ivelina, M.; Charlotte, A.; Naomi, S. J. Org. Chem. 2014, 79, 1947.
[25] Natividad, H.; Maria, S.; Ana, N. J. Agric. Food Chem. 2015, 63, 3681.
[26] Prilezhaeva, E. N.; Shmonina, L. I. Khimicheskaya 1969, 670.
[27] Michitada, S.; Teruya, A.; Watanabe, Y. Yakugaku Zassh 1965, 85, 962.
[28] Murru, S.; Ghosh, H.; Sahoo, K. S.; Patel, K. B. Org. Lett. 2009, 11, 4254.
[29] Prasad, D.; Naidu, A.; Sekar, G. Tetrahedron Lett. 2009, 50, 1411.
[30] Illuminati, G.; Gilman, H. J. Am. Chem. Soc. 1949, 71, 3349.
[31] Fukuzawa, S.; Shimizu, E.; Atsuumi, Y.; Haga, M.; Ogata, K.; Tetrahedron Lett. 2009, 50, 2374.
/
| 〈 |
|
〉 |