

2-苯基/环己基取代环十二酮肟衍生物的合成及晶体结构
收稿日期: 2015-09-21
修回日期: 2015-10-21
网络出版日期: 2015-11-03
基金资助
国家自然科学基金(No. 21172254)资助项目.
Synthesis and Crystal Structure of 2-Phenyl/cyclohexyl Cyclododecane Oxime Derivatives
Received date: 2015-09-21
Revised date: 2015-10-21
Online published: 2015-11-03
Supported by
Project supported by the National Natural Science Foundation of China (No. 21172254).
以环十二酮为原料, 分别与溴代环己烷或苯基锂试剂反应制得2-环己基环十二酮和2-苯基环十二酮, 还意外获得了副产物1,1'-二羟基-1,1'-联环十二烷. 2-环己基环十二酮和2-苯基环十二酮与盐酸羟胺反应制备了2-环己基环十二酮肟和2-苯基环十二酮肟, 与亚硝酸钠-盐酸体系反应获得了3-环己基-1,2-环十二二酮单肟和3-苯基-1,2-环十二二酮单肟. 在溶液中结晶获得其中四个化合物单晶并进行了X射线衍射, 结果表明1-苯基环十二醇和1,1'-二羟基-1,1'-联环十二烷在晶体中的十二元环均采取[3333]构象, 两个取代基在角碳上; 在晶体中2-环己基环十二酮肟的十二元环采取[3333]-2-酮构象, 而3-苯基-1,2-环十二二酮单肟的十二元环则改变为[4233]-3,4-二酮构象, 取代基均在边碳外向位, 量子力学计算的结果与晶体衍射的结果一致. 1,1'-二羟基-1,1'-联环十二烷在晶体中采取摞光盘一样完全重叠的自组装排列方式.
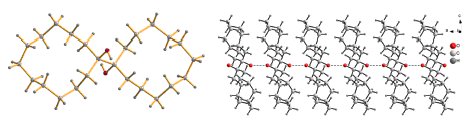
关键词: 环十二酮肟; 1,2-环十二二酮单肟; [3333]构象; [3333]-2-酮; [4233]-3,4-二酮
杨明艳 , 张晓腾 , 王道全 , 王明安 . 2-苯基/环己基取代环十二酮肟衍生物的合成及晶体结构[J]. 有机化学, 2016 , 36(2) : 399 -405 . DOI: 10.6023/cjoc201509025
2-Cyclohexylcyclododecanone and 2-phenylcyclododecanone were synthesized via reaction of cyclododecanone with bromocyclohexane and lithium phenide using cyclododecanone as raw material, respectivey. The 1,1'-dihydroxy-1,1'- bicyclododecane was also obtained unexpected. 2-Cyclohexylcyclododecanone oxime and 2-phenylcyclododecanone oxime were prepared by reaction of 2-cyclohexylcyclododecanone and 2-phenylcyclododecanone with hydroxylamine chloride, 3-cyclohexylcyclododecan-1,2-dione monooxime and 3-phenyl-cyclododecan-1,2-dione monooxime were synthesized by reaction of 2-cyclohexylcyclododecanone and 2-phenylcyclododecanone with NaNO2-HCl system. Their crystals were obtained in the solution and X-ray diffraction analyses were carried out. The results showed that [3333] conformation was took by the cyclododecane ring of 1-phenylcyclododecanol and 1,1'-dihydroxy-1,1'-bicyclododecane, the two substituted groups were at corner position, while 2-cyclohexylcyclododecanone oxime took [3333]-2-one, 3-phenylcyclododecan-1,2-dione monooxime took [4233]-3,4-dione conformation and the substituted groups were at side-exo position in their crystals. The results of quantum mechanics calculation were consistent with those of X-ray diffraction. In the crystal structure of 1,1'-dihydroxy- 1,1'-bicyclododecane, the molecules exhibited unique self-assembly property of complete disc overlapping between two molecular layers in the a-axis direction with intermolecular hydrogen bond.

[1] (a) Zoorob, H. H.; Elsherbini, M. S.; Hamama, W. S. ARKIVOC 2011, 429. (b) Zoorob, H. H.; Elsherbini, M. S.; Hamama, W. S. Am. J. Org. Chem. 2012, 2, 63.
[2] (a) Skibinski, M.; Wang, Y.; Slawin, A. M. Z.; Lebl, T.; Kirsch, P.; O'Hagan, D. Angew. Chem., Int. Ed. 2011, 50, 10581. (b) O'Hagan, D.; Wang Y.; Skibinski M.; Slawin, A. M. Z. Pure Appl. Chem. 2012, 84, 1587. (c) Wang, Y.; Kirsch, P.; Lebl, T.; Slawin, A. M. Z.; O'Hagan, D. Beilstein J. Org. Chem. 2012, 8, 1271.
[3] (a) Sathesh, V.; Umamahesh, B.; Ramachandran, G.; Ravindaranath, S. R.; Sathiyanarayanan, K. I. New J. Chem., 2012, 36, 2292. (b) Sathesh, V.; Natesan, S. K.; Ravindaranath, S. R.; Periyasamy, G.; Sathiyanarayanan, K. I. Med. Chem. Res. 2014, 23, 5086.
[4] Karthikeyan, N. S.; Gunasekar, R.; Sathiyanarayanan, K. I.; Ravindaranath, R. Synth. Commun. 2012, 42, 3429.
[5] Khorasani, S.; Fernandes, M. A.; Perry, C. B. Cryst. Growth Des. 2012, 12, 5908.
[6] (a) Wang, D. Q.; Yang, X. L.; Wang, M. A.; Liang, X. M.; You, T. B. Acta Chim. Sinica 2002, 60, 475 (in Chinese). (王道全, 杨晓亮, 王明安, 梁晓梅, 尤田耙, 化学学报, 2002, 60, 475.) (b) Wang, M. A.; Ma, Z. C.; Lu, H. Z.; Wang, D. Q. Acta Chim. Sinica 2003, 61, 445 (in Chinese). (王明安, 马祖超, 路慧哲, 王道全, 化学学报, 2003, 61, 445.) (c) Wang, M. A.; Ma, Z. C.; Wang D. Q. Acta Chim. Sinica 2003, 61, 399 (in Chinese). (王明安, 马祖超, 王道全, 化学学报, 2003, 61, 399.) (d) Wang, M. A.; Yan, X. J.; Liu, J. P.; Jin, S. H.; Li, T. G.; Yang, X.; Ma, Z. C.; Wang, D. Q. Acta Chim. Sinica 2007, 65, 1657 (in Chinese). (王明安, 闫晓静, 刘建平, 金淑慧, 李太公, 杨旭, 王道全, 化学学报, 2007, 65, 1657.) (e) Wang, M. A.; Zhang, N.; Lu, H. Z.; Wang, D. Q. Chin. J. Chem. 2007, 25, 1196.
[7] (a) Chen, S. C.; Zhang, C. Y.; Zhang, L.; Wang, D. Q.; Wang, M. A. Acta Chim. Sinica 2011, 69, 1354 (in Chinese). (陈守聪, 张春艳, 张莉, 王道全, 王明安, 化学学报, 2011, 69, 1354.) (b) Zhang, C. Y.; Chen, S. C.; Wang, D. Q.; Wang, M. A. Acta Chim. Sinica 2010, 68, 989 (in Chinese). (张春艳, 陈守聪, 王道全, 王明安, 化学学报, 2010, 68, 989.)
[8] Yang, M. Y.; Zhang, L.; Wang, D. Q.; Wang, M. A. Chem. J. Chin. Univ. 2015, 36, 489 (in Chinese). (杨明艳, 张莉, 王道全, 王明安, 高等学校化学学报, 2015, 36, 489.)
[9] (a) McMurry, J. E.; Fleming, M. P. J. Am. Chem. Soc. 1974, 96, 4708. (b) Imamoto, T.; Kusumoto, T.; Hatanaka, Y.; Yokoyama, M. Tetrahedron Lett. 1982, 23, 1353. (c) Mukaiyama, T.; Kagayama, A.; Shiina, I. Chem. Lett. 1998, 11, 1107. (d) Lannou, M.; Helion, F.; Namy, J. Tetrahedron 2003, 59, 10551.
[10] (a) Marshall, J. A.; Black, T. H.; Shone, R. L. Tetrahedron Lett. 1979, 20, 4737. (b) Alex, N.; Pamela, St. J. Z. Tetrahedron Lett. 1980, 21, 3527.
[11] Harpp, D. N.; Steliou, K.; Cheer, C. J. J. Chem. Soc., Chem. Commun. 1980, 825.
[12] Zysman-Colman, E.; Abrams, C. B.; Harpp, D. N. J. Org. Chem. 2003, 68, 7059.
[13] Wang, M. A.; Tu, G. Z.; Ma, Z. C.; Zhang, N.; Wang, D. Q. Chin. J. Chem. 2006, 24, 205.
[14] Li, T. G.; Liu, J. P.; Wang, M. A., J. Shandong Normal Univ. 2009, 24, 72 (in Chinese). (李太公, 刘建平, 王明安, 山东师范大学学报, 2009, 24, 72.)
[15] Alex, N.; Pamela, St. J. Z. J. Org. Chem. 1981, 46, 4685.
[16] Ji, S.; Matsushita, M.; Takahashi, T. T.; Horiuchi, C. A. Tetrahedron Lett. 1999, 40, 6791.
[17] Sheldrick, G. M. Acta Crystallogr. 2008, A64, 112.
[18] Han, X. Y.; Wang, M. A.; Li, T. G.; Liang, X. M.; Dong, Y. H.; Chen, F. H.; Wang, D. Q. Chin. J. Struct. Chem., 2007, 26, 625.
[19] (a) Weinberg, N.; Wolfe, S. J. Am. Chem. Soc. 1994, 116, 9860. (b) Yates, P. C.; Richardson, C. M. THEMOCHEM 1996, 363, 17. (c) Kolossvary, I.; Guida, W. C. J. Am. Chem. Soc. 1993, 115, 2107. (d) Kolossvary, I.; Guida, W. C. J. Am. Chem. Soc. 1996, 118, 5011. (e) Christensen, I. T.; Jorgensen, F. S. J. Comput.-Aided Mol. Des. 1997, 11, 385.
[20] (a) Kolochkov, V. V.; Chernov, P. P.; Polozov, A. M.; Antonovskii, V. L.; Koshel, G. N.; Antonova, T. N.; Aganov, A. V. Zh. Org. Khim. 1990, 26, 387. (b) Ballini, R.; Palestini, C. Tetrahedron Lett. 1994, 35, 5731. (c) Potekhin, K. A.; Maleev, A. V.; Struchkov, Y. T.; Magerramov, A. M.; Kozmin, A. S.; Zefirov, N. S. Dokl. Akad. Nauk 1995, 340, 345. (d) Terent'ev, A. O.; Kutkin, A. V.; Starikova, Z. A. Synthesis 2004, 2356.
[21] Dunitz, J. D.; Shearer, H. M. M. Helv. Chim. Acta 1960, 43, 18.
[22] Rawdah, T. N. Tetrahedron 1991, 47, 8579.
[23] (a) Zhang, Y. F.; Yamato, K.; Zhong, K.; Zhu, J.; Deng, J. G.; Gong, B. Org. Lett. 2008, 10, 4339. (b) Helsel, A. J.; Brown, A. L.; Yamato, K.; Wen, F.; Yuan, L. H.; Clements, A. J.; Harding, S. V.; Szabo, G.; Shao, Z. F.; Gong, B. J. Am. Chem. Soc. 2008, 130, 15784.
/
| 〈 |
|
〉 |