

反-2,3,6(8)-三取代-1,3-苯并噁嗪类化合物的立体选择性合成、晶体结构与杀菌活性
收稿日期: 2015-08-19
修回日期: 2015-10-30
网络出版日期: 2015-11-16
基金资助
国家自然科学基金(Nos. 21042011, 21372070)、国家科技计划支撑子项目(No. 2011BAE06B01)和湖南省高校创新平台开放基金(No. 13k089)资助项目.
Stereoselective Synthesis, Crystal Structure and Fungicidal Activity of trans-2,3,6(8)-Trisubstituted-1,3-benzoxazines
Received date: 2015-08-19
Revised date: 2015-10-30
Online published: 2015-11-16
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21042011, 21372070), the Sub-project of National Science and Technology Supporting Program of China (Nos. 2011BAE06B01) and the Scientific Research Fund of Hunan Provincial Education Department (No. 13K089).
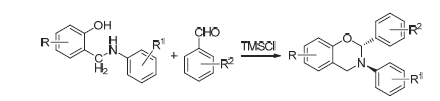
在TMSCl的催化作用下, 2-芳氨基甲基苯酚和取代苯甲醛经N,O-缩醛反应合成了一系列新型反-2,3,6(8)-三取代-1,3-苯并噁嗪类化合物, 收率为38%~80%. 该反应呈现出高度反式选择性. 产物结构用1H NMR, 13C NMR, IR和元素分析等进行了表征. 对目标化合物进行了杀菌活性测试, 在测试浓度下, 大部分显示中等至良好的活性. 其中化合物5d和5f在浓度为50 mg/mL时抗稻瘟病菌的活性达78.6%.
关键词: 2,3,6(8)-三取代-1,3-苯并噁嗪; 合成; 立体选择性; 杀菌活性
唐子龙 , 夏赞稳 , 李新兴 . 反-2,3,6(8)-三取代-1,3-苯并噁嗪类化合物的立体选择性合成、晶体结构与杀菌活性[J]. 有机化学, 2016 , 36(3) : 590 -595 . DOI: 10.6023/cjoc201508019
A series of novel trans-2,3,6(8)-trisubstituted-1,3-benzoxazines were prepared in yields of 38%~80% by reactions of 2-arylaminomethyl phenols with various substituted benzaldehydes in the presence of TMSCl. The reaction showed high trans selectivity. The structures of all the target products were characterized by 1H NMR, 13C NMR, IR and elemental analysis. The fungicidal activities of the prepared compounds were investigated, most of which showed moderate to good activity, compounds 5d and 5f showed growth inhibition of 78.6% against M. oryzae at 50 mg/mL.

[1] Wang, S.; Li, Y.; Liu, Y.; Lu, A.; You, Q. Bioorg. Med. Chem. 2008, 18, 4095.
[2] Chen, S.; Li, X.; Wan, S.; Jiang, T. Synth. Commun. 2012, 42, 2937.
[3] Benameur, L.; Bouaziz, Z.; Nebois, P.; Bartoli, M.; Boltard, M.; Fillion, H. Chem. Pharm. Bull. 1996, 44, 605.
[4] Ihmaid, S. K.; Ai-Rawi, J. M. A.; Bradley, C. J.; Angove, M. J.; Robertson, M. N. Eur. J. Med. Chem. 2012, 57, 85.
[5] Tang, Z.; Xia, Z.; Chang, S.; Wang, Z. Bioorg. Med. Chem. Lett. 2015, 25, 3378.
[6] Konda, S.; Raparthi, S.; Bhaskar, K.; Munaganti, R. K.; Guguloth, V.; Nagarapu, L.; Akkewar, D. M. Bioorg. Med. Chem. Lett. 2015, 25, 1643.
[7] Nemecek, P.; Mocak, J.; Lehotay, J.; Waisser, K. Chem. Papers 2013, 67, 305.
[8] Waisser, K.; Petrlikova, E.; Perina, M.; Klimesova, V.; Kunes, J.; Jr, K. P.; Kaustova, J.; Dahse, H. M.; Mollmann, U. Eur. J. Med. Chem. 2010, 45, 2715.
[9] Pastemak, A.; Goble, S. D.; Struters, M.; Vicario, P. P.; Ayala, J. M.; Salvo, J. D.; Kilbum, R.; Wisniewski, T.; Demartino, J. A.; Mills, S. G.; Yang, L. ACS Med. Chem. Lett. 2010, 1, 14.
[10] Garg, V.; Kumar, A.; Chaudhary, A.; Agrawal, S.; Tomar, P.; Sreenivasan, K. K. Med. Chem. Res. 2013, 22, 5256.
[11] Cobum, C. A.; Meinke, P. T.; Chang, W.; Fandozzi, C. M.; Graham, D. J.; Hu, B.; Huang, Q.; Kargmam, S.; Kozlowski, J.; Liu, R.; Mccauley, J. A.; Nomeir, A. A.; Soll, R. M.; Vacca, J. P.; Wang, D.; Wu, H.; Zhong, B.; Olsen, D. B.; Ludmere, S. W. ChemMedChem 2013, 8, 1930.
[12] Baqar, M.; Agag, T.; Huang, R.; Maia, J.; Qutubuddin, S.; Ishida, H. Macromolecules 2012, 45, 8119.
[13] Kawaguchi, A. W.; Sudo, A.; Endo, T. ACS Macro Lett. 2013, 2, 1.
[14] Deniz, E.; Tomasulo, M.; Sortino, S.; Rayno, F. M. J. Phys. Chem. C 2009, 113, 8491.
[15] Voiciuk, V.; Redeckas, K.; Martynaitis, V.; Steponaviciute, R. A.; Šackus, A.; Vengris, M. J. Photochem. Photobiol. A: Chem. 2014, 278, 60.
[16] Burke, W. J.; Murdock, K. C.; Ec, G. J. Am. Chem. Soc. 1954, 76, 1677.
[17] Mathew, B. P.; Nath, M. J. Heterocycl. Chem. 2009, 46, 1003.
[18] Tang, Z.; Xia, Z.; Ma, H.; Liu, H.; Ou, X. Chin. J. Appl. Chem. 2013, 30, 993 (in Chinese). (唐子龙, 夏赞稳, 马红伟, 刘汉文, 欧晓明, 应用化学, 2013, 30, 993.)
[19] Colin, J. L.; Loubinoux, B. Tetrahedron Lett. 1982, 23, 4245.
[20] Neuvonen, K.; Pihlaja, K. J. Chem. Soc., Perkin Trans. 2 1988, 461.
[21] Mangion, K.; Chen, C.; Li, H.; Maligres, P.; Chen, Y.; Christensen, M.; Cohen, R.; Jeon, I.; Klapars, A.; Krska, S.; Nguyen, H.; Reamer, R. A.; Sherry, B. D.; Zavialov, I. Org. Lett. 2014, 16, 2310.
[22] Richers, M. T.; Breugst, M.; Platonova, A. Y.; Ullrich, A.; Dieckmann, A.; Houk, K. N.; Seidel, D. J. Am. Chem. Soc. 2014, 136, 6123.
[23] Tang, Z.; Chen, W.; Zhu, Z.; Liu, H. Synth. Commun. 2012, 42, 1372.
[24] Tang, Z.; Zhu, Z.; Yan, L.; Chang, S.; Liu, H. J. Heterocycl. Chem. 2013, 50, 1116.
[25] Tang, Z.; Chang, S.; Yan, L.; Cui, M.; Liu, H. Chin. J. Org. Chem. 2012, 32, 1241 (in Chinese). (唐子龙, 常书红, 颜林, 崔美艳, 刘汉文, 有机化学, 2012, 32, 1241.)
[26] Xiao, Z.; Shi, W.; Wang, P.; Wei, W.; Zeng, X.; Zhang, J.; Zhu, N.; Peng, M.; Peng, B.; Lin, X.; Hui, O.; Peng, X.; Wang, G. Bioorg. Med. Chem. 2015, 23, 4508.
[27] Casiraghi, G.; Casnati, G.; Puglia, G.; Sartori, G.; Terenghi, G. J. Chem. Soc., Perkin Trans. 1 1980, 1862.
/
| 〈 |
|
〉 |