

铁催化二级氯代烷烃与炔基格氏试剂的交叉偶联反应
收稿日期: 2015-10-26
修回日期: 2015-12-16
网络出版日期: 2016-01-04
基金资助
葛兰素史克(上海)医药研发有限公司与上海交通大学药学院联合培养项目.
Iron-Catalyzed Cross-Coupling of Secondary Alkyl Chloride and the Alkynes Grignard Reagent
Received date: 2015-10-26
Revised date: 2015-12-16
Online published: 2016-01-04
Supported by
Project supported by the Union-Education Program of Shanghai Jiao Tong University and the Research and Development Center of GlaxoSmithKline.
贾婉 , 赵立志 , 魏恒旭 , 朱林东 , 傅磊 , 陈蔚春 . 铁催化二级氯代烷烃与炔基格氏试剂的交叉偶联反应[J]. 有机化学, 2016 , 36(5) : 1060 -1064 . DOI: 10.6023/cjoc201510031
A green, practical and efficient iron-catalyzed, base free cross-coupling reaction of non-activated secondary alkyl halides with the alkynes Grignard reagent has been developed to afford a variety of alkyl alkynes in high yields, using iron(III) chlorides as catalyst. This approach is quick and easy to operate. It exhibits promising in industry since utilizing the low-cost iron as a catalyst instead of expensive palladium or nickel.
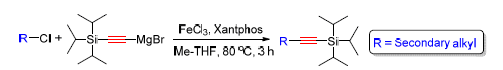
[1] (a) Zhou, J.; Fu, G. C. J. Am. Chem. Soc. 2003, 125, 14726.
(b) Fischer, C.; Fu, G. C. J. Am. Chem. Soc. 2005, 127, 4594.
(c) Arp, F. O.; Fu, G. C. J. Am. Chem. Soc. 2005, 127, 10482.
[2] (a) Saito, B.; Fu, G. C. J. Am. Chem. Soc. 2007, 129, 9602.
(b) Roderiguez, N.; Ramirez de Arellano, C.; Asensio, G.; Medio-Simon, M. Chem. Eur. J. 2007, 13, 4223.
[3] (a) Altenhoff, G.; Wurtz, S.; Glorius, F. Tetrahedron Lett. 2006, 47, 2925.
(b) Caeiro, J.; Perez Sestelo, J.; Sarandeses, L. A. Chem. Eur. J. 2008, 14, 741.
[4] (a) Ikeda, Y.; Nakamura, T.; Yorimitsu, H.; Oshima, K. J. Am. Chem. Soc. 2002, 124, 6514.
(b) Fujioka, T.; Nakamura, T.; Yorimitsu, H.; Oshima, K. Org. Lett. 2002, 4, 2257.
(c) Mizutani, K.; Shinokubo, H.; Oshima, K. Org. Lett. 2003, 5, 3959.
[5] Wang, H.; Li, L.; Xie, X.; Zhang, R.; Liao, B. CN 103498051, 2014 [Chem. Abstr. 2014, 160, 194031].
[6] Sabir, A.; Debasish, K.; Brindaban, C. R. J. Org. Chem. 2014, 79, 7391.
[7] Deepa, K. D.; Selvakannan, P. R.; Patil, S. K.; Choudhary, V. R.; Bhargava, S. K. Appl. Catal. A: Gen. 2014, 476, 54.
[8] (a) Tamura, M.; Kochi, J. K. Synthesis 1971, 303.
(b) Maillard, B.; Forrest, D.; Ingold, K. U. J. Am. Chem. Soc. 1976, 98, 7024.
(c) Thoennes, D.; Wesis, E. Chem. Ber. 1978, 111, 3381.
(d) Zhang, Y.; Feng, B. N. Chin. J. Org. Chem. 2014, 34, 2406 (in Chinese).
(张艳, 冯柏年, 有机化学, 2014, 34, 2406.) (e) Guo, N.; Zhu, S. F. Chin. J. Org. Chem. 2015, 35, 1383 (in Chinese).
(郭娜, 朱守非, 有机化学, 2015, 35, 1383.)
[9] (a) Liu, W.; Lei, A. W. Tetrahedron Lett. 2008, 49, 610.
(b) Liu, W.; Cao, H.; Lei, A. W. Angew. Chem., Int. Ed. 2010, 49, 2004.
(c) Wang, H. B.; Wang, L.; Shang, J. S.; Li, X.; Wang, H. Y.; Gui, J.; Lei, A. W. Chem. Commun. 2012, 48, 76.
[10] (a) Rudolph, A.; Lautens, M. Angew. Chem., Int. Ed. 2009, 48, 2656.
(b) Wenkert, E; Han, A. L.; Jonny, C. J. J. Chem. Soc., Chem. Commun. 1988, 975.
(c) Simon, B. B.; David, W. C. M. J. Am. Chem. Soc. 2003, 125, 6046.
[11] (a) Furstner, A.; Leitner, A.; Mendez, M.; Krause, H. J. Am. Chem. Soc. 2002, 124, 13856.
(b) Kuzmina, M. O.; Steib, K. A.; Flubacher, D.; Knochel, P. Org. Lett. 2012, 14(18).
(c) Kuzmina, M. O.; Steib, K. A.; Markiewicz, T. J.; Flubacher, D. Angew. Chem., Int. Ed. 2013, 52, 4945.
(d) Nakamura, E.; Yoshikai, N. J. Org. Chem. 2010, 75, 6061.
[12] Hatakeyama, T.; Okada, Y.; Yoshimoto, Y.; Nakamura, M. Angew. Chem., Int. Ed. 2011, 50, 10973.
[13] Cheung, C. W.; Ren, P.; Hu, X. Org. Lett. 2014, 16, 2566.
[14] Zhang, C. X.; Li, N. N.; Li, X.; Chang, H. H.; Liu, Q.; Wei, W. L. Chin. J. Org. Chem. 2014, 34, 81 (in Chinese).
(张聪霞, 李娜娜, 李兴, 常宏宏, 刘强, 魏文珑, 有机化学, 2014, 34, 81.)
[15] (a) Tan, M. X.; Gu, Y. Q.; Luo, X. J.; Zhang, P. Chin. J. Org. Chem. 2015, 35, 781 (in Chinese).
(谭明雄, 顾运琼, 罗旭建, 张培, 有机化学, 2015, 35, 781.) (b) Zhang, B.; Guan, H. X.; Liu, B.; Shi, B. F. Chin. J. Org. Chem. 2014, 34, 1487 (in Chinese).
(张博, 管晗曦, 刘斌, 史炳锋, 有机化学, 2014, 34, 1487.) (c) Zhang, W. M.; Dai, J. J.; Xu, H. J. Chin. J. Org. Chem. 2015, 35, 1820 (in Chinese).
(张文曼, 戴建军, 许建华, 有机化学, 2015, 35, 1820.)
[16] Eiichi, N.; Takuji, H.; Shingo, I.; Kentaro, I.; Laurean, L.; Nakamura, M. Organic Reactions, Vol. 83, John Wiley & Sons, Inc., Japan, 2014.
[17] (a) Nakamura, M.; Hirai, A.; Nakamura, E. J. Am. Chem. Soc. 2000. 122, 978.
(b) Yamagami, T.; Shintani, R. T. Org. Lett. 2007, 9, 1045.
[18] (a) Nakamura, M.; Matsuo, K.; Inoue, T. Org. Lett. 2003, 5, 1373.
(b) Lautens, M.; Fagnous, K.; Hiebert, S. Acc. Chem. Res. 2003, 36, 48.
/
| 〈 |
|
〉 |