

三苯基铋全氟辛基磺酸盐催化β-氨基醇高效合成
收稿日期: 2015-10-29
修回日期: 2016-01-21
网络出版日期: 2016-01-29
基金资助
国家自然科学基金(No.21373003)、湖南省自然科学基金(No.14JJ7027)、湖南省教育厅重点(No.15A041)资助项目.
Efficient Synthesis of β-Amino Alcohols Promoted by Triphenylbismuth Bisperfluorooctanesulfonates
Received date: 2015-10-29
Revised date: 2016-01-21
Online published: 2016-01-29
Supported by
Projected support by the National Natural Science Foundation of China (No. 21373003), the Natural Science Foundation of Hunan Province (No. 14JJ7027), and the Key Project of Hunan Province Education Department (No. 15A041).
谭年元 , 王鹏辉 , 许新华 , 高青松 , 区泽棠 , 邱仁华 . 三苯基铋全氟辛基磺酸盐催化β-氨基醇高效合成[J]. 有机化学, 2016 , 36(5) : 1094 -1098 . DOI: 10.6023/cjoc201510035
In this paper, a novel air-stable triphenylbismuth bisperfluorooctanesulfonate was synthesized by treatment of moisture-sensitive triphenylbismuth dichloride with silver bisperfluorooctanesulfonate in anhydrous CH2Cl2 at room temperature. Its catalytic assessment results show that the reactions of arylamine and epoxide proceed efficiently in the presence of triphenylbismuth bisperfluorooctanesulfonates 1(5.0 mol%) under solvent-free condition, affording β-amino alcohols in good to excellent yields. The yields of desired products do not reduce obviously after recycling for 4 times of catalyst 1. Hence, a convenient and efficient method for preparation of β-amino alcohols is provided.
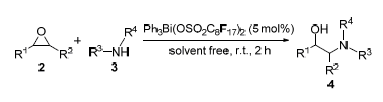
[1] Métro, T.-X.; Appenzeller, J.; Pardo, D. G.; Cossy, J. Org. Lett. 2006, 8, 3509.
[2] Métro, T.-X.; Pardo, D. G.; Cossy, J. J. Org. Chem. 2007, 72, 6556.
[3] Tan, W.; Zhao, B.-X.; Sha, L.; Jiao, P.-F.; Wan, M.-S.; Su, L. Chin. J. Org. Chem. 2005, 25, 1011 (in Chinese).
(谭伟, 赵宝祥, 沙磊, 焦培福, 万茂生, 苏磊, 有机化学, 2005, 25, 1011.)
[4] Job, G. E.; Buchwald, S. L. Org. Lett. 2002, 4, 3703.
[5] Tanaka, Y.; Taniguchi, N.; Uemura, M. Org. Lett. 2002, 4, 835.
[6] Liu, W.-M.; Wang, P.-A.; Zhang, S.-Y.; Jiang, R. Chin. J. Org. Chem. 2006, 26, 518 (in Chinese).
(柳文敏, 王平安, 张生勇, 姜茹, 有机化学, 2006, 26, 518.)
[7] Pan, S. Q.; Zhang, C. H.; Li, Y.; Yan, S. J.; Lin, J. Chin. J. Org. Chem. 2011, 31, 193 (in Chinese).
(潘圣强, 张从海, 李烨, 严胜骄, 林军, 有机化学, 2011, 31, 193.)
[8] Yang, F. F.; Gu, X. F.; Yang, L.; Gao, Y. H.; Lu, H. F. Fine Chem. 2013, 30, 956 (in Chinese).
(杨飞飞, 顾晓峰, 杨林, 高玉华, 陆鸿飞, 精细化工, 2013, 30, 956.)
[9] Reddy, L. R.; Reddy, M. A.; Bhanumathi, N.; Rao, K. R. New J. Chem. 2001, 25, 221.
[10] Ollevier, T.; Lavie-Compin, G. Tetrahedron Lett. 2002, 43, 7891.
[11] Thirupathaiah, A.; Ramanna, S.; Rao, G. V. Orient. J. Chem. 2009, 25, 165.
[12] Chakraborti, A. K.; Kondaskar, A. Tetrahedron Lett. 2003, 44, 8315.
[13] Swamy, N. R.; Goud, T. V.; Reddy, S. M.; Krishnaiah, P.; Venkateswarlu, Y. Synth. Commun. 2004, 34, 727.
[14] Singh, M. C.; Peddinti, R. K. Tetrahedron Lett. 2007, 48, 7354.
[15] Pachón, L. D.; Gamez, P.; Brussel. J. J. M.; Reedijk, J. Tetrahedron Lett. 2003, 44, 6025.
[16] Kamal, A.; Prasad, B. R.; Reddy, A. M.; Khan, M. N. A. Catal. Lett. 2007, 8, 1876.
[17] Yadav, J. S.; Reddy, B. V. S.; Basak, A. K.; Venkat Narsaiah, A. Tetrahedron Lett. 2003, 44, 1047.
[18] Azizi, N.; Saidi, M. R. Tetrahedron 2007, 63, 888.
[19] Kureshy, R. I.; Singh, S.; Khan, N. H.; Abdi, S. H. R.; Suresh, E.; Jasra, R. V. J. Mol. Catal. A: Chem. 2007, 264, 162.
[20] Sreedhar, B.; Radhika, P.; Neelima, B.; Hebalkar, N. J. Mol. Catal. A: Chem. 2007, 272, 159.
[21] Bordoloi, A.; Hwang, Y. K.; Hwang, J-S.; Halligudi, S. B. Catal. Commun. 2009, 10, 1398.
[22] Danafar, H.; Yadollahi, B. Catal. Comm. 2009, 10, 842.
[23] Heravi, M. M.; Bakhtiari, K.; Alinejhad, H.; Saeedi, M.; Malakooti, R. Chin. J. Chem. 2010, 28, 269.
[24] Yin, S. F.; Maruyama, J.; Yamashita, T.; Shimada, S. Angew. Chem., Int. Ed. 2008, 47, 6590.
[25] De, S. K.; Gibbs, R. A. Tetrahedron Lett. 2005, 46, 8345.
[26] Thirupathaiah, A.; Rao, G. V. Indian J. Chem. 2008, 47B, 1762.
[27] Hollis, T. K.; Robison, N. P.; Bosnich, B. Tetrahedron Lett. 1992, 33, 6423.
[28] Li, X. S.; Kurita, A.; Man-E, S.; Orita, A.; Otera, J. Organometallics 2005, 24, 2567.
[29] An, D. L.; Peng, Z. H.; Orita, A.; Kurita, A.; Man-e, S.; Ohkubo, K.; Li, X. S.; Fukuzumi, S.; Otera, J. Chem. Eur. J. 2006, 12, 1642.
[30] Qiu, R. H.; Xu, X. H.; Peng, L. F.; Zhao, Y. L.; Li, N. B.; Yin, S. F. Chem. Eur. J. 2012, 18, 6172.
[31] Li, N. B.; Wang, J. Y.; Zhang, X. H.; Qiu, R. H.; Wang, X.; Chen, J. Y.; Yin, S. F.; Xu, X. H. Dalton Trans. 2014, 43, 11696.
[32] Zhang, X. H.; Qiu, R. H.; Zhou, C. C.; Yu, J. X.; Li, N. B.; Yin, S. F.; Xu, X. H. Tetrahedron 2015, 71, 1011.
[33] Placzek, A. T.; Donelson, J. L.; Trivedi, R.; Gibbs, R. A.; De, S. K. Tetrahedron Lett. 2005, 46, 9029.
[34] Wang, P. H.; Wang, J. Y.; Au, C.-T.; Qiu, R. H.; Xu, X. H.; Yin, S. F. Adv.Synth. Catal.2016, 358, 1302.
[35] Simona, B.; Francesco, F.; Ferdinando, P.; Luigi, V. Synlett 2007, 2683.
[36] Qiu, R. H.; Wang, P. H.; Wang, J. Y.; Zhu, L. Z.; Yin, S. F.; Xu, X. H.CN 105131041, 2015.
[37] Danafar, H.; Yadollahi, B. Catal. Commun. 2009, 10, 842.
/
| 〈 |
|
〉 |