

Ugi反应脱Boc保护基环合策略在含氮杂环合成中的应用
收稿日期: 2015-11-03
修回日期: 2016-01-07
网络出版日期: 2016-02-01
基金资助
国家自然科学基金(No.30873140)、北京市优秀人才培养(No.20071D0501600227)、北京市教委科技发展(No.KM201010028011)资助项目.
Application of Ugi/De-Boc/Cyclization Strategy in the Synthesis of Nitrogen-Containing Heterocycles
Received date: 2015-11-03
Revised date: 2016-01-07
Online published: 2016-02-01
Supported by
Project supported by the National Natural Science Foundation of China (No. 30873140), the Program for Excellent Talents of Beijing City (No. 20071D0501600227) and the Beijing Municipal Commission of Education (No. KM201010028011).
张钊瑞 , 郑晓霖 , 郭长彬 . Ugi反应脱Boc保护基环合策略在含氮杂环合成中的应用[J]. 有机化学, 2016 , 36(6) : 1241 -1265 . DOI: 10.6023/cjoc201511004
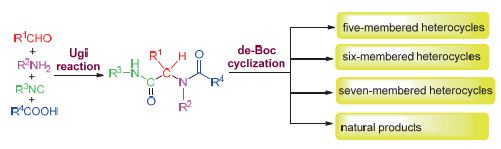
Ugi/de-Boc/cyclization (UDC) strategy represents a “three-step, one-pot procedure”, employing the Ugi multi-component reaction utilizing one bi-functional group substrates with N-Boc-protected amino, followed by Boc-deprotection and cyclization to synthesize nitrogen-containing heterocycles. This strategy has the characteristics of rapid, efficiency, economy and structural diversity, which can be used to build libraries of complex nitrogen-containing heterocycles and has drawn much attention recently. In this paper, the research progress of UDC strategy in the synthesis of five-, six- and seven-mem- bered heterocycles and natural products were summarized.
[1] Domling, A. Chem. Rev. 2006, 106, 17.
[2] Zhang, S. S.; Wen, R. H.; Yang, X. Y. Multicomponent Reactions, Chemical Industry Press, Beijing, 2008, p. 1 (in Chinese). (张书圣, 温永红, 杨晓燕, 多组分反应, 化学工业出版社, 北京, 2008, p. 1.)
[3] Zhang, X.-N.; Li, Y.-X.; Zhang, Z.-H. Tetrahedron 2010, 67, 7426.
[4] Schwerkoske, J.; Masquelin, T.; Perun, T.; Hulme, C. Tetrahedron Lett. 2005, 46, 8355.
[5] Boomhoff, M.; Yadav, A. K.; Appun, J.; Schneider, C. Org. Lett. 2014, 16, 6236.
[6] Kalinski, C.; Umkehrer, M.; Weber, L.; Kolb, J.; Burdack, C.; Ross, G. Mol. Diversity 2010, 14, 513.
[7] Yan, N.; Xia, J. H.; Xiong, Y. K.; Xiong, B.; Lin, C. H.; Liao, W. L. Chin. J. Org. Chem. 2014, 34, 2487 (in Chinese). (严楠, 夏剑辉, 熊云奎, 熊斌, 林春花, 廖维林, 有机化学, 2014, 34, 2487.)
[8] Sun, J.; Shen, G. L.; Xie, Y. J.; Yan, C. G. Chin. J. Chem. 2014, 32, 1143.
[9] Guo, R.-Y.; An, Z.-M.; Mo, L.-P.; Wang, R.-Z.; Liu, H.-X.; Wang, S.-X.; Zhang, Z.-H. ACS Comb. Sci. 2013, 15, 557.
[10] Ugi, I.; Meyr, R.; Fetzer, U. Angew. Chem. 1959, 71, 386.
[11] Ugi, I. Steinbrückner, C. Angew. Chem. 1960, 72, 267.
[12] Ugi, I. Angew. Chem., Int. Ed. 1962, 1, 8.
[13] Kalinski, C.; Umkehrer, M.; Gonnard, S.; Jager, N.; Ross, G.; Hiller, W. Tetrahedron Lett. 2006, 47, 2041.
[14] Gordillo-Cruz, R. E.; Renteria-Gomez, A.; Islas-Jacome, A.; Cortes-Garcia, C. J.; Diaz-Cervantes, E.; Robles, J.; Gamez-Mon- tano, R. Org. Biomol. Chem. 2013, 11, 6470.
[15] Zeng, X.-H.; Wang, H.-M.; Ding, M.-W. Org. Lett. 2015, 17, 2234.
[16] Barlow, T. M. A.; Jida, M.; Tourwe, D.; Ballet, S. Org. Biomol. Chem. 2014, 12, 6986.
[17] Peshkov, A. A.; Peshkov, V. A.; Pereshivko, O. P.; Van der Eycken, E. V. Tetrahedron 2015, 71, 3863.
[18] Saha, D.; Wadhwa, P.; Sharma, A. RSC Adv. 2015, 5, 33067.
[19] Yerande, S. G.; Newase, K. M.; Singh, B.; Boltjes, A.; Domling, A. Tetrahedron Lett. 2014, 55, 3263.
[20] Hulme, C.; Peng, J.; Tang, S. Y.; Burns, C. J.; Morize, I.; Labaudiniere, R. J. Org. Chem. 1998, 63, 8021.
[21] Parks, D. J.; LaFrance, L. V.; Calvo, R. R.; Milkiewicz, K. L.; Gupta, V.; Lattanze, J.; Ramachandren, K.; Carver, T. E.; Petrella, E. C.; Cummings, M. D.; Maguire, D.; Grasberger, B. L.; Lu, T. Bioorg. Med. Chem. Lett. 2005, 15, 765.
[22] Ma, N.; L, Z.-M.; Z, W.-G. Prog. Chem. 2003, 15, 186 (in Chinese).
(马宁, 李正名, 赵卫光, 化学进展, 2003, 15, 186.)
[23] Hulme, C.; Dietrich, J. Mol. Diversity 2009, 13, 195.
[24] Zhang, W.; Tempest, P. Tetrahedron Lett. 2004, 45, 6757.
[25] Dai, W.-M.; Shi, J, Y. Comb. Chem. High Throughput Screening 2007, 10, 837.
[26] Hulme, C.; Gore, V. Curr. Med. Chem. 2003, 10, 51.
[27] Hulme, C.; Ma, L.; Cherrier, M. P.; Romano, J. J.; Morton, G.; Duquenne, C.; Salvino, J.; Labaudiniere, R. Tetrahedron Lett. 2000, 41, 1883.
[28] Hulme, C.; Ma, L.; Romano, J. J.; Morton, G.; Tang, S. Y.; Cherrier, M. P.; Choi, S.; Salvino, J.; Labaudiniere, R. Tetrahedron Lett. 2000, 41, 1889.
[29] Hulme, C.; Ma, L.; Romano, J.; Morrissette, M. Tetrahedron Lett. 1999, 40, 7925.
[30] Zhan, P.; Chen, X. W.; Li, D. Y.; Fang, Z. J.; De Clercq, E.; Liu, X. Y. Med. Chem. Commun. 2013, 33, S1.
[31] Huang, S.-T.; Hsei, I.-J.; Chen, C. Bioorg. Med. Chem. 2006, 14, 6106.
[32] Goodwin, K. D.; Lewis, M. A.; Tanious, F. A.; Tidwell, R. R.; David, W. W.; Georgiadis, M. M.; Long, E. C. J. Am. Chem. Soc. 2006, 128, 7846.
[33] Lin, Y.-Z.; Wang, T.-H.; Lin, Y.-S.; Kuan, W.-C.; Lee, W.-C. J. Appl. Polym. Sci. 2014, 131, 40725.
[34] Xiong, J.-F.; Luo, S.-H.; Huo, J.-P.; Liu, J.-Y.; Chen, S.-X.; Wang, Z.-Y. J. Org. Chem. 2014, 79, 8366.
[35] Tempest, P.; Ma, V.; Thomas, S.; Hua, Z.; Kelly, M. G.; Hulme, C. Tetrahedron Lett. 2001, 42, 4959.
[36] Carbajales, C.; Prado, M. A.; Gutierrez-de-Teran, H.; Cores, A.; Azuaje, J.; Novio, S.; Nunez, M. J.; Fernandez-Garcia, B.; Sotelo, E.; Garcia-Mera, X.; Sanchez-Lazo, P.; Freire-Garabal, M.; Coelho, A. ChemBioChem 2014, 15, 1471.
[37] Tempest, P.; Ma, V.; Kelly, M. G.; Jones, W.; Hulme, C. Tetrahedron Lett. 2001, 42, 4963.
[38] Xu, Z. G.; Shaw, A. Y.; Dietrich, J.; Cappelli, A. P.; Nichol, G.; Hulme, C. Mol. Diversity 2012, 16, 73.
[39] Shaw, A. Y.; Medda, F.; Hulme, C. Tetrahedron Lett. 2012, 53, 1313.
[40] Xu, Z. G; Ayaz, M.; Cappelli, A. A.; Hulme, C. ACS Comb. Sci. 2012, 14, 460.
[41] Xu, Z. G.; Shaw, A. Y.; Nichol, G. S.; Cappelli, A. P.; Hulme, C. Mol. Diversity 2012, 16, 607.
[42] Xu, Z, G.; Martinez-Ariza, G.; Cappelli, A. P.; Roberts, S. A. J. Org. Chem. 2015, 80, 9007.
[43] Song, G.-T.; Li, S.-Q.; Yang, Z.-W.; Yuan, J.-H.; Wang, M.-S.; Zhu, J.; Chen Z.-Z.; Xu, Z.-G. Tetrahedron Lett. 2015, 56, 4616.
[44] Ozkay, Y.; Tunal, Y.; Karaca, H.; Ilhan, I. Eur. J. Med. Chem. 2010, 45, 3293.
[45] Chen, Z.-Z.; Zhang, J.; Tang, D.-Y.; Xu, Z.-G. Tetrahedron Lett. 2014, 55, 2742.
[46] Hulme, C.; Chappeta, S.; Griffith, C.; Lee, Y.-S.; Dietrich, J. Tetrahedron Lett. 2009, 50, 1939.
[47] Nixey, T.; Kelly, M.; Semin, D.; Hulme, C. Tetrahedron Lett. 2002, 43, 3681.
[48] Medda, F.; Martinez-Ariza, G.; Hulme, C. Tetrahedron Lett. 2015, 56, 5295.
[49] Davenport, A. J.; Stimson, C. C.; Corsi, M.; Vaidya, D.; Glenn, E.; Jones, T. D.; Bailey, S.; Gemkow, M. J.; Fritz, U.; Hallett, D. J. Bioorg. Med. Chem. Lett. 2010, 20, 5165.
[50] Meanwell, N. A. J. Med. Chem. 2011, 54, 2529.
[51] Gunawan, S.; Hulme, C. Org. Biol. Chem. 2013, 11, 6036.
[52] Gunawan, S.; Petit, J.; Hulme, C. ACS Comb. Sci. 2012, 14, 160.
[53] Gunawan, S.; Nichol, G.; Hulme, C. Tetrahedron Lett. 2012, 53, 1664.
[54] Cao, S.; Zhong, S. S.; Hu, C. F.; Wan, J.-P.; Wen, C. P. Chin. J. Chem. 2015, 33, 568.
[55] Xu, Z. G.; Moliner, F. D.; Cappelli, A. P.; Hulme, C. Angew. Chem., Int. Ed. 2012, 51, 8037.
[56] Achour, R.; Essassi, E. M.; Zniber, R. Tetrahedron Lett. 1988, 29, 195.
[57] Hulme, C.; John, P.; Louridas, B.; Menard, P.; Krolikowski, P.; Kumar, N. V. Tetrahedron Lett. 1998, 39, 8047.
[58] Xu, Z. G.; Moliner, F. D.; Cappelli, A. P.; Ayaz, M.; Hulme, C. Synlett 2014, 25, 225.
[59] Houston, D. R.; Synstad, B.; Eijsink, V. G. H.; Stark, M. J. R.; Eggleston, I. M.; van Aalten, M. F. J. Med. Chem. 2004, 47, 5713.
[60] Abraham, W.-R. Drug Des. Rev. Online 2005, 2, 13.
[61] Van de Merwe, E.; Huang, D.; Peterson, D.; Kilian, G.; Milne, P. J.; Van de Vanter, M.; Frost, C. Peptides. 2008, 29, 1305.
[62] Rhoden, C. R. B.; Rivera, D. G.; Kreye, O.; Bauer, A. K.; Westermann, B.; Wessjohann, L. A. J. Comb. Chem. 2009, 11, 1078.
[63] Hulme, C.; Cherrier, M.-P. Tetrahedron Lett. 1999, 40, 5295.
[64] Ji, F.; Yi, W. B.; Cai, C. J. Heterocycl. Chem. 2014, 51, 1287.
[65] Hulme, C.; John, P.; Morton, G.; Salvino, J. M.; Herpin, T.; Labaudiniere, R. Tetrahedron Lett. 1998, 39, 7227.
[66] Raillard, S. P.; Ji, G. J.; Mann, A. D.; Baer, T. A. Org. Process Res. Dev. 1999, 3, 177.
[67] Golebiowski, A.; Jozwik, J.; Klopfenstein, S. R.; Colson, A. O.; Grieb, A. L.; Russell, A. F.; Rastogi, V. L.; Diven, C. F.; Portlock, D. E.; Chen, J. J. J. Comb. Chem. 2002, 4, 584.
[68] Cano-Herrera, M.-A.; Miranda, L. D. Chem. Commun. 2011, 47, 10770.
[69] Nixey, T.; Tempest, P.; Hulme, C. Tetrahedron Lett. 2002, 43, 1637.
[70] Dalvi, P. B.; Lin, S.-F.; Paike, V.; Sun, C.-M. ACS Comb. Sci. 2015, 17, 421.
[71] Ayaz, M.; Guillermo, M. A.; Hulme, C. Synlett 2014, 25, 1680.
[72] Azuaje, J.; Maatougui, A. E.; Garcia-Mera, X.; Sotelo, E. ACS Comb. Sci. 2014, 16, 403.
[73] Ayaz, M.; Xu, Z. G.; Hulme, C. Tetrahedron Lett. 2014, 55, 3406.
[74] Gunawan, S.; Nichol, G.; Hulme, C. Tetrahedron Lett. 2012, 53, 1664.
[75] Zhang, Z.-H.; Zhang, X.-N.; Mo, L.-P.; Li, Y.-X.; Ma, F.-P. Green Chem. 2012, 14, 1502.
[76] Zhang, Z.-H.; Lu, H.-Y.; Yang, S.-H.; Gao, J.-W. J. Comb. Chem. 2010, 12, 643.
[77] Noolvi, M. N.; Patel, H. M.; Bhardwaj, V.; Chauhan, A. Eur. J. Med. Chem. 2011, 46, 2327.
[78] Youssef, K.; Nadine, A.; Aurelien, D.; Sebastien, H.; Michele, L.; Pierre, V.; Armand, G.; Patrice, V. Eur. J. Med. Chem. 2010, 45, 616.
[79] Tiwari R.; Chhabra, G. Asian J. Chem. 2010, 22, 5981.
[80] Dietrich, J.; Kaiser, C.; Meurice, N.; Hulme, C. Tetrahedron Lett. 2010, 51, 3951.
[81] Azuaje, J.; Maatougui, A. E.; Perez-Rubio, J. M.; Coelho, A.; Fernandez, F.; Sotelo, E. J. Org. Chem. 2013, 78, 4402.
[82] Qneibi, M. S.; Micale, N.; Grasso, S.; Niu, L. Biophys. J. 2013, 104, 1405.
[83] Qneibi, M. S.; Micale, N.; Grasso, S.; Niu, L. Biochemistry 2012, 51, 1787.
[84] Anzini, M.; Valenti, S.; Braile, C.; Cappelli, A.; Vomero, S.; Alcaro, S.; Ortuso, F.; Marinelli, L.; Limongelli, V.; Novellino, E.; Betti, L.; Giannaccini, G.; Lucacchini, A.; Daniele, S.; Martini, C.; Ghelardini, C.; Mannelli, L. D.; Giorgi, G.; Mascia, M. P.; Biggio, G. J. Med. Chem. 2011, 54, 5694.
[85] Liu, P.; Lanza, T. J.; Chioda, M.; Jones, C.; Chobanian, H. R.; Guo, Y.; Chang, L.; Kelly, T. M.; Kan, Y.; Palyha, O.; Guan, X.-M.; Marsh, D. J.; Metzger, J. M.; Ramsay, K.; Wang, S.-P.; Strack, A. M.; Miller, R.; Pang, J.; Lyons, K.; Dragovic, J.; Ning, J. G.; Schafer, W. A.; Welch, C. J.; Gong, X.; Gao, Y.-D.; Hornak, V.; G. Ball, R.; Tsou, N.; Reitman, M. L.; Wyvratt, M. J.; Nargund, R. P.; Lin, L. S. ACS Med. Chem. Lett. 2011, 2, 933.
[86] Huang, Y.; Wolf, S.; Bista, M.; Meireles, L.; Camacho, C.; Holak, T. A.; Domling, A. Chem. Biol. Drug Des. 2010, 76, 116.
[87] Huang, Y.; Domling, A. Chem. Biol. Drug Des. 2010, 76, 130.
[88] Huang, Y.; Khoury, K.; Chanas, T.; Domling, A. Org. Lett. 2012, 14, 5916.
[89] Gordon, C. P.; Young, K. A.; Hizartzidis, L.; Deane, F. M.; McCluskey, A. Org. Biomol. Chem. 2011, 9, 1419.
[90] Tempest, P.; Pettus, L.; Gore, V.; Hulme, C. Tetrahedron Lett. 2003, 44, 1947.
[91] Azuaje, J.; Perez-Rubio, J. M.; Yaziji, V.; Maatougui, A. E.; Gonzalez-Gomez, J. C.; Sanchez-Pedregal, V. M.; Navarro-Vazquez, A.; Masaguer, C. F.; Teijeira, M.; Sotelo, E. J. Org. Chem. 2015, 80, 1533.
[92] Huang, Y.; Khoury, K.; Chanas, T.; Domling, A. Org. Lett. 2012, 14, 5916.
[93] Hulme, C.; Ma, L.; Kumar, N. V.; Krolikowski, P. H.; Allen, A. C.; Labaudiniere, R. Tetrahedron Lett. 2000, 41, 1509.
[94] Zhou, H.; Zhang, W.; Yan, B. J. Comb. Chem. 2010, 12, 206.
[95] Gunawan, S.; Nichol, G. S.; Chappeta, S.; Dietrich, J.; Hulme, C. Tetrahedron Lett. 2010, 51, 4689.
[96] Gunawan, S.; Ayaz, M.; Moliner, F. D.; Frett, B.; Kaiser, C.; Patrick, N.; Xu, Z.; Hulme, C. Tetrahedron 2012, 68, 5606.
[97] Xu, Z. G.; Dietrich, J.; Shaw, A. Y.; Hulme. C. Tetrahedron Lett. 2010, 51, 4566.
[98] Brown, A. L.; Churches, Q. I.; Hutton, C. A. J. Org. Chem. 2015, 80, 9831.
[99] Boger, D. L.; Saionz, K. W. Bioorg. Med. Chem. 1999, 7, 315.
[100] Katayama, K.; Nakagawa, K.; Takeda, H.; Matsuda, A.; Ichikawa, S. Org. Lett. 2014, 16, 428.
/
| 〈 |
|
〉 |