

α,β-不饱和羰基化合物的不对称Diels-Alder反应研究进展
收稿日期: 2016-01-20
修回日期: 2016-03-01
网络出版日期: 2016-03-28
基金资助
武警后勤学院博士启动基金(No.WHB201506)和武警后勤学院创新团队科学基金(No.WHTD201303)资助项目.
Progress on the Asymmetric Diels-Alder Reaction of α,β-Unsaturated Carbonyl Compounds
Received date: 2016-01-20
Revised date: 2016-03-01
Online published: 2016-03-28
Supported by
Project supported by the Doctoral Operation Foundation of Medical College of PAP (No. WHB201506) and the Innovative Research Team Program for Scienec and Technology in Logistics University of PAP (No. WHTD201303).
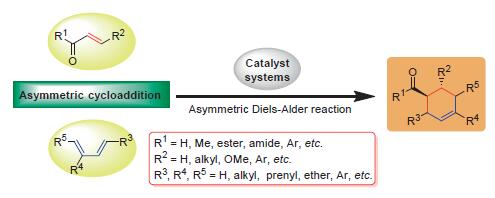
Diels-Alder反应是典型的[4+2]环合反应,自被发现以来备受化学家们的重视. 不对称Diels-Alder反应是合成手性中间体的有效手段,在手性药物和天然产物全合成中得到广泛应用. α,β-不饱和羰基化合物是有机合成反应的关键中间体,根据其结构特点和催化体系类型,分类阐述了α,β-不饱和羰基化合物为亲双烯体的不对称正电子需求Diels-Alder反应的研究进展.
关键词: 不对称Diels-Alder反应; 天然产物; 有机合成反应; α,β-不饱和羰基化合物
刘文香 , 吴宇强 , 李灵芝 , 李霞 . α,β-不饱和羰基化合物的不对称Diels-Alder反应研究进展[J]. 有机化学, 2016 , 36(7) : 1501 -1512 . DOI: 10.6023/cjoc201601027
Diels-Alder reaction is a [4+2] concerted cycloaddition reaction, which has attracted considerable attention since its discovery. Asymmetric Diels-Alder reaction is an effective method for synthesis of chiral intermediates, so it was widely applied in the total synthesis of drugs and natural products. α,β-Unsaturated carbonyl compounds are a kind of highly reactive versatile intermediates in organic synthesis, according to its structural characteristics and catalytic types, recent advances in asymmetric normal electron-demand Diels-Alder reaction of α,β-unsaturated carbonyl compounds are reviewed in this paper.

[1] Diels, O.; Alder, K. Ann. 1928, 460, 98.
[2] Wang, C.; Liu, W.-H.; Zhou, X.; Li, Y.-M.; Li, Y-.P. Prog. Chem. 2009, 9, 1857 (in Chinese). (王春, 刘伟华, 周欣, 李越敏, 李云鹏, 化学进展, 2009, 9, 1857.)
[3] (a) Yates, P. J. Am. Chem. Soc. 1960, 82, 4436.
(b) Walborsky, H. M.; Barash, L.; Davis, T. C. J. Org. Chem. 1961, 26, 4778.
(c) Walborsky, H. M.; Barash, L.; Davis, T. C. Tetrahedron 1963, 19, 2333.
[4] Jeffrey, D.W. Chem. Rev. 1996, 96, 167.
[5] Wang, S.-M.; Li, Y.; Guo, R.-X.; Li, L.-G.; Chang, H.-P.; Li, Z.; Shi, Q.-W. Nat. Prod. Res. Devel. 2013, 25, 698 (in Chinese). (王思明, 郭瑞霞, 李力更, 常和平, 李骘, 史清文, 李勇, 天然产物研究与开发, 2013, 25, 698.)
[6] Dennis, E. D. Clin. Pharmacol. Ther. 1986, 40, 125.
[7] (a) Lan, Z.-W.; Li, Y.; Chen, H.; Xiao, Y.-F. Organic Chemistry, Ocean Press, Beijing, 2004 (in Chinese). (蓝仲薇, 李瑛, 陈华, 肖友发, 有机化学, 海洋出版社, 北京, 2004.)
(b) Ou, Y.-Y.; Yu, H.-F.; Dong, D.-W.; Liu, Q. J. Northeast Normal. Univ. 2006, 38, 67 (in Chinese). (欧阳艳, 于海丰, 董德文, 刘群, 东北师大学报, 2006, 38(1), 67.)
(c) Fuchs, N.; Augustin, M.; Humam, M.; Alexakis, A.; Taras, R.; Gladiali, S. Tetrahedron 2005, 16, 3143.
[8] (a) Liu, W.-M.; Wang, Q.-F.; Zhang, S.-Y. Chin. J. Org. Chem. 2007, 27, 862 (in Chinese). (柳文敏, 王巧峰, 张生勇, 有机化学, 2007, 27, 862.)
(b) Mendelovici, M.; Glotter, E. J. Chem. Soc., Perkin Trans. 1 1992, 1735.
[9] (a) Pei, W. J. Yanbian Univ. 1997, 23, 29 (in Chinese). (裴文, 延边大学学报, 1997, 23, 29.)
(b) Pei, W.; Xun, M. -Z.; Sun, L.; Ni, Z.-M.; Piao, H.-R. Chin. J. Synth. Chem. 2002, 10, 49 (in Chinese). (裴文, 徐明哲, 孙莉, 倪哲明,朴虎日, 合成化学, 2002, 10, 49.)
(c) Pei, W.; Sun, L.; Wang H.-B.; Deng, Q.; Sun, L. Chin. J. Org. Chem. 2006, 26(5), 727 (in Chinese). (裴文, 孙莉, 王海滨, 邓琼,孙莉, 有机化学, 2006, 26(5), 727.)
[10] (a) Xu, K.-X. Refined Chemical Raw Materials and Intermediate Manual, Chemical Industry Press, Beijing, 1998 (in Chinese). (徐克勋, 精细有机化工原料及中间体手册, 化学工业出版社, 北京, 1998.)
(b) Sun, B.-G.; He, J. Perfume Chemistry Process, Chemical Industry Press, Beijing, 2004 (in Chinese). (孙保国, 何坚, 香料化学与工艺, 化学工业出版社, 北京, 2004.)
[11] Woodward, R.; Hoffmann, R. J. Am. Chem. Soc. 1965, 87, 395.
[12] Fukui, K.; Yonezawa, T.; Shingu, H. J. Chem. Phys. 1952, 20, 722.
[13] Corey, E. J.; Ensley, H. E. J. Am. Chem. Soc. 1975, 97, 6908.
[14] Evans, D.; Chapman, K.; Bisaha, J. J. Am. Chem. Soc. 1984, 106, 4261.
[15] Oppolzer, W.; Christian,C.; Gerald, B. Preliminary Commun. 1984, 67, 1397.
[16] Enholm, E. J.; Jiang, S. J. Org. Chem. 2000, 65, 4756.
[17] Bañuelos, P.; García, J. M.; Enrique, G. B.; Palomo, C.; Herrero, A.; Odriozola, J. M.; Oiarbidet, M.; Razkin, J. J. Org. Chem. 2010, 75, 1458.
[18] David, S.; Eustache, J.; Lubineau, A. J. Chem. Soc., Perkin Trans. 1 1974, 2274.
[19] Bednarski, M.; Danishefsky, S.; J. Am. Chem. Soc. 1983, 105, 6968.
[20] (a) Gupta, R. C.; Harland, P. A.; Stoodley, R. J. J. Chem. Soc. Chem. Commun. 1983, 754.
(b) Gupta, R. C.; Harland, P. A.; Stoodley, R. J. Tetrahedron 1984, 40, 4657.
(c) Gupta, R. C.; Slawin, A. M. Z.; Stoodley, R. J.; Williams, D. J. J. Chem. Soc. Chem. Commun. 1986, 668.
(d) Gupta, R. C.; Slawin, A. M. Z.; Stoodley, R. J.; Williams, D. J. J. Chem. Soc., Chem. Commun. 1986, 1116.
(e) Gupta, R. C.; Raynor, C. M.; Stoodley, R. J.; Slawin, A. M. Z.; Williams, D. J. J. Chem. Soc., Perkin Trans. 1 1988,1773.
(f) Gupta, R. C.; Larsen, D. S.; Stoodley, R. J.; Slawin, A. M. Z.; Williams, D. J. J. Chem. Soc., Perkin Trans. 1 1989, 739.
[21] Kozmin, S. A.; Rawal, V. H. J. Am. Chem. Soc. 1999, 121, 9562.
[22] (a) Davis, F. A.; JenkinsJr, R. H.; Awad, S. B.; Stringer, O. D.; Watson, W. H.; Galloy, J. J. Am. Chem. Soc. 1982, 104, 5412.
(b) Kagan, H, B.; Riant, O. Chem. Rev. 1992, 92, 1007.
(c) Dias, L, C. J. Brazilian Chem. Soc. 1997, 8, 289.
(d) Ahrendt, K. K.; Borths, C. J.; Macmillan, D. W. C. J. Am. Chem. Soc. 2000, 122, 4243.
(e) Li, G.-L.; Liang, T.; Wojtas, L.; Antilla, J. C. Angew. Chem., Int. Ed. 2013, 52, 4628.
(f) Hatano, M.; Hayashi, K.; Sakamoto, T.; Makino, Y.; Ishihara, K. Synlett 2016, 27, A-G.
[23] Corey, E. J.; Weinshenker, N. M.; Schaaf, T. K. J. Am. Chem. Soc. 1969, 91, 5675.
[24] Corey, E. J.; Imwinkelried, R.; Pikul, S. J. Am. Chem. Soc. 1989, 111, 5493.
[25] Corey, E. J.; Loh, T. P. J. Am. Chem. Soc. 1991, 113, 8966.
[26] Corey, E.J.; Ishihara, K. Tetrahedron Lett. 1992, 33, 6807.
[27] Hayashi, Y.; Rohde, J. J.; Corey, E. J. J. Am. Chem. Soc. 1996, 118, 5502.
[28] Hu, Q. Y.; Rege, P. D.; Corey, E. J. J. Am. Chem. Soc. 2004, 126, 5984.
[29] Chapuis, C.; Jurczak, J. Helv. Chim. Acta 1987, 70, 436.
[30] Ishihara, J.; Nakadachi, S.; Watanabe, Y.; Hatakeyama, S. J. Org. Chem. 2015, 80, 2037.
[31] Narasaka, K.; Iwasawa, N.; Inoue, M.; Yamada, T.; Nakashima, M.; Sugimori, J. J. Am. Chem. Soc. 1989, 111, 5340.
[32] Corminboeuf, O.; Renaud, P. Org. Lett. 2002, 4, 1735.
[33] Orimoto, K.; Oyama, H.; Namera, Y.; Niwa, T.; Nakada, M. Org. Lett. 2013, 15, 768
[34] Harada, S.; Morikawa, T.; Nishida, A. Org Lett. 2013, 15, 5314.
[35] Nicolaou, K. C.; Snyder, S. A.; Montagnon, T.; Vassilikogiannakis, G. Angew. Chem., Int. Ed. 2002, 41, 1668.
[36] (a) Noyori, R. Adv. Synth. Catal. 2001, 343, 1.
(b) Noyori, R. Chem. Commun. 2005, 1807.
[37] (a) Helmchen, G.; Pfaltz, A. Acc. Chem. Res. 2000, 33, 336.
(b) Zhang, W.; Chi, Y.; Zhang, X. Acc. Chem. Res. 2007, 40, 1278.
(c) Chan, A. S. C.; Hu, W.; Pai, C. C.; Lau, C. P.; Jiang, Y.; Mi. A.; Yan, M.; Sun, J.; Lou, R.; Deng, J. J. Am. Chem. Soc. 1997, 119, 9570.
(d) Wu, J.; Chan, A. S. C. Acc. Chem. Res. 2006, 39, 711.
(e) Xie, J. H.; Zhou, Q. L. Acc. Chem. Res. 2008, 41, 581.
(f) Ding, K.; Han, Z.; Wang, Z. Chem.-Asian J. 2009, 4, 32.
(g) Han, Z.; Wang, Z.; Zhang, X.; Ding, K. Angew. Chem., Int. Ed. 2009, 48, 5345.
(h) Wang, D.; Hu, X.; Huang, J.; Deng, J.; Yu, S.; Duan, Z.; Xu, X.; Zheng, Z. Angew. Chem., Int. Ed. 2007, 46, 7810.
(i) Zhou, J.; Tang, Y. J. Am. Chem. Soc. 2002, 124, 9030.
[38] Merino, P.; Marqués-López, E.; Tejero, T.; Herrera, R, P. Synthesis 2010, 1.
[39] Hine, J.; Linden, S. M.; Kanagasabapathy, V. M. J. Org. Chem. 1985, 50, 5096.
[40] Kelly, T. R.; Meghani, P.; Ekkundi, V. S. Tetrahedron Lett. 1990, 31, 3381.
[41] Huang, Y.; Unni, A. K.; Thadani, A. N.; Rawal, V. H. Nature 2003, 424, 146.
[42] Thadani, A. N.; Stankovic, A. R.; Rawal, V. H. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5846.
[43] Li, C.; Yu, X., Lei, X. Org. Lett. 2010, 12, 4284.
[44] (a) Liu, H.; Cun, L. F.; Mi, A. Q.; Jiang, Y. Z.; Gong, L. Z. Org. Lett. 2006, 8, 6023.
(b) Akiyama, T.; Tamura, Y.; Itoh, J. Synlett 2006, 141.
[45] Han, Z.-Y.; Chen, D.-F.; Wang, Y.-Y.; Guo, R.; Wang, P.-S.; Wang, C.; Gong, L.-Z. J. Am. Chem. Soc. 2012, 134, 6532.
[46] Wang, Y.; Tu, M.-S., Lei, Y., Sun, M.; Shi, F. J. Org. Chem. 2015, 80, 3223.
[47] (a) Momiyama, N.; Konno, T.; Furiya, Y.; Iwamoto, T.; Terada, M. J. Am. Chem. Soc. 2011, 133, 19294.
(b) Wu, H.; He, Y.-P.; Shi, F. Synthesis 2015, 47, 1990.
[48] Nakashima, D.; Yamamoto, H. J. Am. Chem. Soc. 2006, 128, 9626.
[49] Schuster, T.; Bauch, M.; Dürner, G.; Gobel, M. W. Org Lett. 2000, 2, 179.
[50] Marko, W.; Gerd, D.; Bats, J. W.; Gobel, M. W. J. Org. Chem. 2010, 75, 2718.
[51] (a) Zheng, C.-W.; Lu, Y.-P.; Zhang, J.-K.; Chen, X.-K.; Chai, Z.; Ma, W.-Y.; Zhao, G. Chemistry 2010, 16, 5853.
(b) Singh, R. P.; Bartelson, K.; Wang, Y.; Su, H.; Lu, X.; Deng, L. J. Am. Chem. Soc. 2008, 130, 2422.
(c) Chen, H. -T.; Trewyn, B. G.; Wiench, J. W.; Pruski, M.; Lin, V. S. Y. Top. Catal. 2010, 53, 187.
(d) Siau, W. Y.; Wang, J. Catal. Sci. Technol. 2011, 1, 1298.
(e) Jiang, X.-X.; Shi, X.-M.; Wang, S.-L.; Sun, T.; Cao, Y.-M.; Wang, R. Angew. Chem., Int. Ed. 2012, 51, 2084.
(f) Mao, Z.-J.; Lin, A.-J.; Shi, Y.; Mao, H.-B.; Li, W.-P.; Cheng, Y.-X.; Zhu, C.-J. J. Org. Chem. 2013, 78, 10233.
(g) Held, F. E.; Tsogoeva, S. B. Catal. Sci. Technol. 2016, 6, 645.
[52] Tan, B.; Hernandez-Torres, G.; Barbas, C. F. J. Am. Chem. Soc. 2011, 133, 12354.
[53] Baum, J. S.; Viehe, H. G. J. Org. Chem. 1976, 41, 183.
[54] Ahrendt, K. A.; Borths, C. J.; Macmillan, D. W. C. J. Am. Chem. Soc. 2000, 122, 4243.
[55] Northrup, A. B.; Macmillan, D. W. C. J. Am. Chem. Soc. 2002, 124, 2458.
[56] Abbasov, M. E.; Hudson, B. M.; Tantillo, D. J.; Romo, D. J. Am. Chem. Soc. 2014, 136, 4492
[57] Kelly, T. R.; Whiting, A.; Chandrakumar, N. S. J. Am. Chem. Soc. 1986, 108, 3510.
[58] Maruoka, K.; Sakurai, M.; Fujiwara, J.; Yamamoto, H. Tetrahedron Lett. 1986, 27, 4895.
[59] Wipf, P.; Jung, J. K. J. Org. Chem. 2000, 65, 6319.
[60] (a) Nomura, T.; Hano, Y. Nat. Prod. Rep. 1994, 11, 205.
(b) Nomura, T. Pure Appl. Chem. 1999, 71, 1115.
(c) Nomura, T.; Hano, Y.; Ueda, S. Stud. Nat. Prod. Chem. 1995, 17, 451.
(d) Nomura, T. Pure Appl. Chem. 1999, 71, 1115.
[61] Corbett, J. L.; Weavers, R. T. Synth. Commun. 2008, 38, 489.
[62] (a) Jung, E. M.; Lee, Y. R. Bull. Korean Chem. Soc. 2008, 29, 1199.
(b) Chee, C. F.; Abdullah, I.; Buckle, M. J. Tetrahedron Lett. 2010, 51, 495.
(c) Chee, C. F.; Lee, Y. K.; Buckle, M. J. C; Rahman, N. A. Tetrahedron Lett. 2011, 52, 1797.
(d) Gunawan, C.; Rizzacasa, M. A. Org. Lett. 2010, 12, 1388.
[63] Cong, H.; Ledbetter, D.; Rowe, G. T.; Caradonna, J. P.; Porco, Jr. J. A. J. Am. Chem. Soc. 2008, 130, 9214.
[64] Cong, H.; Becker, C. F.; Elliott, S. J.; Grinstaff, M. W.; Porco,Jr. J. A. J. Am. Chem. Soc. 2010, 132, 7514.
[65] Boonsri, S.; Gunawan, C.; Krenske, E. H. Rizzacasa. M. A. Org. Biomol. Chem. 2012, 10, 6010.
[66] Han, J.; Jones, A. X.; Lei, X. Synthesis 2014, 47.
[67] Li, X.; Han, J.; Jones, A. X.; Lei, X. J. Org. Chem. 2016, 8, 458.
[68] (a) Han, J.; Li, X.; Guan, Y.; Zhao, W.; Wulff, W.; Lei, X. Angew. Chem. Int. Ed. 2014, 53, 9257.
(b) Lei, G.; Han, J.; Lei, X. Org. Lett., 2016, 18, 360.
[69] (a) Pingfan, Li.; Yamamoto. H. J. Am. Chem. Soc. 2009, 131, 16628.
(b) Gademann, K.; Chavez, D. E.; Jacobsen, E. N. Angew. Chem., Int. Ed. 2002, 41, 3185.
(c) Jiang, X.-X.; Wang, R. Chem. Rev. 2013, 113, 5515.
[70] Wei, W.-D. Organic Chemical Raw Materials, Chemical Industry Press, Beijing, 1999 (in Chinese). (魏文德, 有机化工原料大全(中卷), 化学工业出版社, 北京, 1999.)
/
| 〈 |
|
〉 |