

芳酰胺类衍生物的合成及蛋白酪氨酸磷酸酶1B和含SH2结构域蛋白酪氨酸磷酸酶2抑制活性研究
收稿日期: 2016-03-28
修回日期: 2016-04-27
网络出版日期: 2016-05-17
基金资助
国家自然科学基金(No.21472069)和中国科学院上海药物研究所新药研究国家重点实验室(No.SIMM1302KF-05)资助项目.
Synthesis of Aromatic Amide Derivatives and Their Biological Evaluation against Protein Tyrosine Phosphatase 1B and Scr Homology-2 Domain Containing Protein Tyrosine Phosphatase-2
Received date: 2016-03-28
Revised date: 2016-04-27
Online published: 2016-05-17
Supported by
Project supported by the National Natural Science Foundation of China (No.21472069) and the State Key Laboratory of Drug Research (No.SIMM1302KF-05).
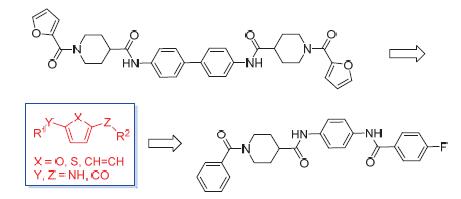
为拓展含串联二芳酰胺结构的蛋白酪氨酸磷酸酶1B(PTP1B)抑制剂的化学空间,将其中的联苯二胺结构单元简化为芳基酰胺结构单元,设计并合成了18个芳酰胺类化合物.活性测试结果表明,部分芳酰胺衍生物对PTP1B和含SH2结构域蛋白酪氨酸磷酸酶2(SHP2)显示了一定强度的抑制活性.其中化合物3c[IC50=(5.13±0.21)μmol/L]对PTP1B显示了中等强度的抑制活性,并且对其他亚型[T细胞蛋白酪氨酸磷酸酶(TCPTP)、含SH2结构域蛋白酪氨酸磷酸酶1(SHP1)和SHP2]显示了一定的选择性.有意思的是,化合物12对SHP2显示了中等强度的抑制活性[IC50=(7.47±1.26)μmol/L],对PTP1B、TCPTP以及SHP1显示了2倍的选择性,为发现新型选择性SHP2抑制剂提供了新的骨架类型.
王文龙 , 骆欢 , 高雅 , 高立信 , 盛丽 , 周宇波 , 李佳 , 李静雅 , 冯柏年 . 芳酰胺类衍生物的合成及蛋白酪氨酸磷酸酶1B和含SH2结构域蛋白酪氨酸磷酸酶2抑制活性研究[J]. 有机化学, 2016 , 36(9) : 2142 -2149 . DOI: 10.6023/cjoc201603045
To explore the chemical space of protein tyrosine phosphatase 1B (PTP1B) inhibitors by changing bis-aromatic amide moiety into aromatic amide moiety, a series of aromatic amide derivatives were designed, synthesized and their biological activities were evaluated against PTP1B and Scr homology-2 domain containing protein tyrosine phosphatase-2 (SHP2). Among them, compound 3c displayed moderate inhibitory activity with IC50 of (5.13±0.21) μmol/L against PTP1B and showed two times selectivity for other related PTPs. Interestingly, compound 12[IC50=(7.47±1.26) μmol/L] showed moderate inhibitory activity against SHP2 and 2-fold selectivity against PTP1B, T-cell protein tyrosine phosphatase (TCPTP) or Src homology-2 domain containing protein tyrosine phosphatase-1 (SHP1) respectively, and offered a novel scaffold to develop new SHP2 inhibitors.

Key words: PTP1B; SHP2; aromatic amide derivatives; inhibitors
[1] Tonks,N.K.FEBS J.2013,280,346.
[2] Hendriks,W.J.;Elson,A.;Harroch,S.;Pulido,R.;Stoker,A.;den Hertog,J.FEBS J.2013,280,708.
[3] Nunes-Xavier,C.E.;Martín-Pérez,J.;Elson,A.;Pulido,R.Biochim.Biophys.Acta,Rev.Cancer 2013,1836,211.
[4] Barr,J.Future Med.Chem.2010,2,1563.
[5] Shi,D.;Li,J.;Jiang,B.;Guo,S.;Su,H.;Wang,T.Bioorg.Med.Chem.Lett.2012,22,2827.
[6] Liu,J.;Jiang,F.;Jin,Y.;Zhang,Y.;Liu,J.;Liu,W.;Fu,L.Eur.J.Med.Chem.2012,57,10.
[7] Xie,J.;Tian,J.;Su,L.;Huang,M.;Zhu,X.;Ye,F.;Wan,Y.Bioorg.Med.Chem.Lett.2011,21,4306.
[8] Qian,S.;Li,H.;Chen,Y.;Zhang,W.;Yang,S.;Wu,Y.J.Nat.Prod.2010,73,1743.
[9] Zhang,Y.;Zhang,W.;Hong,D.;Shi,L.;Shen,Q.;Li,J.-Y.;Li,J.;Hu,L.-H.Bioorg.Med.Chem.2008,16,8697.
[10] Li,H.;Wang,S.-C.;Li,Q.-L.;Song,Y.-W.;Liu,X.-Q.Prog.Modern Biomed.2015,15,1595(in Chinese). (李辉,王曙辰,李庆腊,宋玉文,刘晓谦,现代生物医学进展,2015,15,1595.)
[11] Hu,Z.-Q.;Zhang,C.-M.Transl.Med.J.2013,2,113(in Chi-nese). (胡忠倩,张炽敏,转化医学杂志,2013,2,113.)
[12] Gu,J.;Han,T.;Zhao,N.;Xie,X.-D.;Guo,X.Chin.J.Cancer Biother.2013,20,623(in Chinese). (谷佳,韩涛,赵宁,谢晓冬,郭星,中国肿瘤生物治疗杂志,2013,20,623.)
[13] Butterworth,S.;Overduin,M.;Barr,A.J.Future Med.Chem.2014,6,1423.
[14] Munter,S.D.;Kohn,M.;Bollen,M.ACS Chem.Biol.2013,8,36.
[15] Wang,W.-L.;Huang,C.;Gao,L.-X.;Tang,C.-L.;Wang,J.-Q.;Wu,M.-C.;Sheng,L.;Chen,H.-J.;Nan,F.-J.;Li,J.-Y.;Li,J.;Feng,B.Bioorg.Med.Chem.Lett.2014,24,1889.
[16] Carrilho,R.M.B.;Almeida,A.R.;Kiss,M.;Kollár,L.;Skoda-Földes,R.;D?browski,J.M.;Moreno,M.J.S.M.;Pereira,M.M.Eur.J.Org.Chem.2015,1840.
[17] Konovalova,S.A.;Avdeenko,A.P.;Santalova,A.A.;Pala-marchuk,G.V.;D'yakonenko,V.V.;Shishkin,O.V.Russ.J.Org.Chem.2015,51,42.
[18] Zhang,L.;Xu,W.Y.;Chang,G.J.CN 102604095,2012[Chem.Abstr.2012,157,295637].
[19] Wang,K.L.;Li,X.G.;Liu,F.Y.;Zhang,H.F.;Li,X.Y.;Mao,C.X.;Zhang,J.J.;Wang,R.;Wang,G.J.;Dai,Y.Q.;Wang,K.;Zhang,H.Y.;Gong,Y.L.CN 103539692,2014[Chem.Abstr.2014,160,294897].
[20] Sellarajah,S.;Lekishvili,T.;Bowring,C.;Thompsett,A.R.;Rudyk,H.;Birkett,C.R.;Brown,D.R.;Gilbert,I.H.J.Med.Chem.2004,47,5515.
[21] Kazutoshi,O.;Takeshi,S.WO 2014077324,2014[Chem.Abstr.2014,160,740581].
[22] Miller,R.E.;Reid,W.A.Exp.Parasitol.1986,61,359.
[23] Urbanski,J.;Manek,M.B.Pol.J.Chem.1983,57,603.
[24] Coles,S.J.;Gale,P.A.;Hursthouse,M.B.;Light,M.E.;Warriner,C.N.Supramol.Chem.2004,16,469.
[25] Wang,W.-L.;Yang,D.-L.;Gao,L.-X.;Tang,C.-L.;Ma,W.-P.;Ye,H.-H.;Zhang,S.-Q.;Zhao,Y.-N.;Xu,H.-J.;Hu,Z.;Chen,X.;Fan,W.-H.;Chen,H.-J.;Li,J.-Y.;Nan,F.-J.;Li,J.;Feng.B.Molecules 2013,19,102.
[26] Wang,W.-L.;Chen,X.;Gao,L.-X.;Sheng,L.;Li,J.-Y.;Li,J.;Feng,B.Chem.Biol.Drug Des.2015,86,1161.
[27] Shi,L.;Yu,H.-P.;Zhou,Y.-Y.;Du,J.-Q.;Shen,Q.;Li,J.-Y.;Li,J.Acta.Pharmacol.Sin.2008,29,278.
/
| 〈 |
|
〉 |