

吲哚并四氢呋喃-咪唑盐杂合物的合成及细胞毒活性研究
收稿日期: 2016-10-28
修回日期: 2017-01-12
网络出版日期: 2017-01-20
基金资助
国家自然科学基金(Nos.21662043,21462049,U1402227,21332007)、长江学者和创新团队发展计划(No.IRT13095)、云南省自然科学基金(No.2013FA028)资助项目.
Synthesis and Cytotoxic Activity of Novel Hybrid Compounds between Indolo[b]tetrahydrofuran and Imidazolium Salts
Received date: 2016-10-28
Revised date: 2017-01-12
Online published: 2017-01-20
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21662043, 21462049, U1402227, 21332007), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13095), the Natural Science Foundation of Yunnan Province (No. 2013FA028).
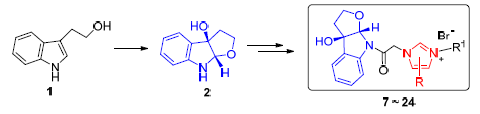
从色醇出发,通过Sharpless环氧化、酰胺化、偶联和成盐四步反应合成了一系列新型的吲哚并四氢呋喃-咪唑盐杂合物,其结构经1H NMR,13C NMR,HRMS以及X射线单晶衍射确定.对合成的新化合物进行了体外抗肿瘤细胞毒活性筛选,结果表明,1-((3aR,8aS)-3,3a-二氢-3a-羟基-2H-呋喃并[2,3-b]吲哚-8(8aH)-基)乙酮-3-(2-(萘-2-基)-2-氧乙基)-5,6-二甲基-1H-苯并[d]咪唑-3-溴盐(20)和1-((3aR,8aS)-3,3a-二氢-3a-羟基-2H-呋喃并[2,3-b]吲哚-8(8aH)-基)乙酮-3-(2-萘甲基))-5,6-二甲基-1H-苯并[d]咪唑-3-溴盐(22)具有较好的体外肿瘤生长抑制活性,对SMMC-7721、MCF-7和SW-480肿瘤细胞株的活性均优于顺铂(DDP),1-((3aR,8aS)-3,3a-二氢-3a-羟基-2H-呋喃并[2,3-b]吲哚-8(8aH)-基)乙酮-3-(2-溴苄基))-5,6-二甲基-1H-苯并[d]咪唑-3-溴盐(24)对SW-480肿瘤细胞株表现出较好的选择性细胞毒活性,其IC50值约为顺铂的2.0倍.
刘正芬 , 张朝波 , 段胜祖 , 刘洋 , 陈文 , 李艳 , 张洪彬 , 羊晓东 . 吲哚并四氢呋喃-咪唑盐杂合物的合成及细胞毒活性研究[J]. 有机化学, 2017 , 37(6) : 1506 -1515 . DOI: 10.6023/cjoc201610043
A series of novel hybrid compounds between indolo[b]tetrahydrofuran and imidazolium salts were prepared from tryptophol by four steps of Sharpless epoxidation, amidation, coupling and salt formation. Their structures were confirmed by 1H NMR, 13C NMR, HRMS and X-ray crystallographic analysis. These compounds were evaluated in vitro against a panel of human tumor cell lines. The results showed that 1-((3aR,8aS)-3,3a-dihydro-3a-hydroxy-2H-furo[2,3-b]indol-8(8aH)-yl)etha-none-3-(2-(naphthalen-2-yl)-2-oxoethyl)-5,6-dimethyl-1H-benzo[d]-imidazol-3-ium bromide (20) and 1-((3aR,8aS)-3,3a-di-hydro-3a-hydroxy-2H-furo[2,3-b]indol-8(8aH)-yl)ethanone-3-(2-naphthylmethyl)-5,6-dimethyl-1H-benzo[d]imidazol-3-ium bromide (22) exhibited higher inhibitory activity selectively against SMMC-7721, MCF-7 and SW480 cell lines compared with DDP. In particular, 1-((3aR,8aS)-3,3a-dihydro-3a-hydroxy-2H-furo[2,3-b]indol-8(8aH)-yl)ethanone-3-(2-bromobenzyl)-5,6-dimethyl-1H-benzo[d]imidazol-3-ium bromide (24) was more selective to SW-480 cell lines with IC50 values 2.0-fold lower than DDP.

[1] Pan, C. X.; Guan, Y. F.; Zhang, H. B. Chin. J. Org. Chem. 2012, 32, 1116 (in Chinese).(潘成学, 关一富, 张洪彬, 有机化学, 2012, 32, 1116.)
[2] Wang, X. Q.; Li, Y.; Yang, X. D.; Zhang, H. B. Chin. J. Org. Chem. 2015, 35, 1276 (in Chinese).(汪学全, 李艳, 羊晓东, 张洪彬, 有机化学, 2015, 35, 1276.)
[3] (a) Suzuki, T.; Choi, J. H.; Kawaguchi, T.; Yamashita, K.; Morita, A.; Hirai, H.; Nagai, K.; Hirose, T.; Omura, S.; Sunazuka, T.; Kawagishi, H. Bioorg. Med. Chem. Lett. 2012, 22, 4246.
(b) Chaudhaery, S. S.; Roy, K. K.; Shakya, N.; Saxena, G.; Sammi, S. R.; Nazir, A.; Nath, C.; Saxena, A. K. J. Med. Chem. 2010, 53, 6490.
(c) Luo, W.; Yu, Q. S.; Kulkarni, S. S.; Parrish, D. A.; Holloway, H. W.; Tweedie, D.; Shafferman, A.; Lahiri, D. K.; Brossi, A.; Greig, N. H. J. Med. Chem. 2006, 49, 2174.
(d) Gavuzzo, E.; Pomponi, M. J. Biochem. Mol. Toxicol. 2002, 16, 64.
[4] (a) Hayashi, M.; Kim, Y.-P.; Takamatsu, S.; Enomoto, A.: Shinose, M.; Takahashi, Y.; Tanaka, H.; Komiyama, K.; Ohmura, S. J. Antibiot. 1996, 49, 1091.
(b) Takamatsu, S.; Kim, Y.-P.; Enomoto, A.; Hayashi, M.; Tanaka, H.; Komiyama, K.; Ohmura, S. J. Antibiot. 1997, 50, 1069.
[5] Hayashi, M.; Rho, M.-C.; Enomoto, A.; Fukami, A.; Kim, Y.-P.; Kikuchi, Y.; Sunazuka, T.; Hirose, T.; Komiyama, K.; Ohmura, S. Proc. Natl. Acad. Sci. 2002, 99, 14728.
[6] (a) Strassmann, G.; Masui, Y.; Chizzonite, R.; Fong, M. J. Immunol. 1993, 150, 2341.
(b) Zhang, X. G.; Bataille, R.; Jourdan, M.; Saeland, S.; Banchereau, J.; Mannoni, P.; Klein, B. Blood 1990, 76, 2599.
[7] Wan, L.; Tius, M. A. Org. Lett. 2007, 9, 647.
[8] (a) Vlahakis, J. Z.; Lazar, C.; Crandall, I. E.; Szarek, W. A. Bioorg. Med. Chem. 2010, 18, 6184.
(b) Dominianni, S. J.; Yen, T. T. J. Med. Chem. 1989, 32, 2301.
(c) Pardin, C.; Schmitzer, A. R.; Leclercq, L. Chem. Eur. J. 2010, 16, 4686.
[9] (a) Fortuna, C. G.; Barresi, V.; Berellini, G..; Musumarra, G. Bioorg. Med. Chem. 2008, 16, 4150.
(b) Saberi, M. R.; Vinh, T. K.; Yee, S. W.; Griffiths, B. J. N.; Evan, P. J.; Simsons, C. J. Med. Chem. 2006, 49, 1016.
[10] Cui, B.; Zheng, B. L.; He, K.; Zheng, Q. Y. J. Nat. Prod. 2003, 66, 1101.
[11] (a) Zeng, X. H.; Yang, X. D.; Zhang, Y. L.; Qing, C.; Zhang, H. B. Bioorg. Med. Chem. Lett. 2010, 20, 1844.
(b) Yang, X. D.; Zeng, X. H.; Zhang, Y. L.; Qing, C.; Song, W. J.; Li, L.; Zhang, H. B. Bioorg. Med. Chem. Lett. 2009, 19, 1892.
[12] D'hooghe, M.; Mollet, K.; De Vreese, R.; Jonckers, T. H. M.; Dams, G.; De Kimpe, N. J. Med. Chem. 2012, 55(11), 5637.
[13] Viegas-Junior, C.; Danuello, A.; Bolzani, V. S.; Barreiro, E. J.; Fraga, C. A. M. Curr. Med. Chem. 2007, 14(17), 1829.
[14] (a) Walsh, J. J.; Bell, A. Curr. Pharm. Des. 2009, 15, 2970.
(b) Zhu, P. F.; Zhao, J. F.; Yang, X. D.; Zhang, H. B. Chin. J. Org. Chem. 2014, 34, 1167 (in Chinese).(朱培芳, 赵静峰, 羊晓东, 张洪彬, 有机化学, 2014, 34, 1167.)
[15] (a) Zhou, B.; Liu, Z. F.; Deng, G. G.; Chen, W.; Li, M. Y.; Yang, L. J.; Li, Y.; Yang, X. D., Zhang, H. B. Org. Biomol. Chem. 2016, 14, 9423.
(b) Zhou, Y. J.; Duan, K. Y.; Zhu, L.; Liu, Z. F.; Zhang, C. G.; Yang, L. J.; Li, M. Y.; Zhang, H. B., Yang, X. D. Bioorg. Med. Chem. Lett. 2016, 26, 460.
(c) Liu, L. X.; Wang, X. Q.; Zhou, B.; Yang, L. J.; Li, Y.; Zhang, H. B.; Yang, X. D. Sci. Rep. 2015, 5, 13101.
(d) Xu, X. L.; Yu, C. L.; Chen, W.; Li, Y. C.; Yang, L. J.; Li, Y.; Zhang, H. B.; Yang, X. D. Org. Biomol. Chem. 2015, 13, 1550.
(e) Xu, X. L.; Wang, J.; Yu, C. L.; Chen, W.; Li, Y. C.; Li, Y.; Zhang, H. B.; Yang, X. D. Bioorg. Med. Chem. Lett. 2014, 24, 4926.
(f) Sun, C. J.; Chen, W.; Li, Y.; Liu, L. X.; Wang, X. Q.; Li, L. J.; Zhang, H. B.; Yang, X. D. RSC Adv., 2014, 4, 16312.
(g) Liu, L. X.; Wang, X. Q.; Yan, J. M.; Li, Y.; Sun, C. J.; Chen, W.; Zhou, B.; Zhang, H. B.; Yang, X. D. Eur. J. Med. Chem. 2013, 66, 423.
(h) Wang, X. Q.; Liu, L. X.; Li, Y.; Sun, C. J.; Chen, W.; Li, L.; Zhang, H. B.; Yang, X. D. Eur. J. Med. Chem. 2013, 62, 111.
[16] Tomoyasu, H.; Toshiaki, S.; Daisuke, Y.; Naoto, K.; Tatsuya, S.; Yoshihiro, H.; Isao, K.; Satoshi, O. Tetrahedron 2005, 61, 6015.
[17] CCDC 1509927 and 1509928 contain the supplementary crystallographic data for compounds 11 and 18. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
/
| 〈 |
|
〉 |