

含吡啶环的3-苯基-1-丙酮肟醚的合成及生物活性
收稿日期: 2017-01-04
修回日期: 2017-02-12
网络出版日期: 2017-02-27
基金资助
山东省自然科学基金(No.ZR2014BM030)资助项目.
Synthesis and Biological Activity of Novel 3-Phenylpropan-1-one Oxime Ethers Containing Pyridine Moiety
Received date: 2017-01-04
Revised date: 2017-02-12
Online published: 2017-02-27
Supported by
Project supported by the Natural Science Foundation of Shandong Province (No. ZR2014BM030).
昝宁宁 , 万福贤 , 王士春 , 张君辉 , 姜林 . 含吡啶环的3-苯基-1-丙酮肟醚的合成及生物活性[J]. 有机化学, 2017 , 37(6) : 1537 -1541 . DOI: 10.6023/cjoc201701009
To search for the pyridine pesticide lead compound with high antigungal activity, a series of novel 3-phenyl propan-1-one oxime ethers bearing pyridine moiety were synthesized using 3-acetypyridine, benzaldehyde, substituted anilines and alkoxyamine hydrochlorides as starting materials. The structures of target compounds were determined by IR, 1H NMR, 13C NMR and elemental analysis. Meanwhile, the target compounds were evaluated for their antifungal activities against S. sclerotiorum and B. cinerea by the mycelium growth rate method. The results indicated that some compounds displayed high antifungal activity to the two kinds of pathogenic fungi, and their activities were even higher than that of the positive control, chlorothalonil.
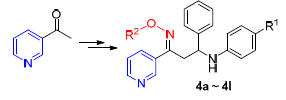
Key words: heterocycles; pyridine; ketones; oxime ethers; biological activitity
[1] Alexander, F. P.; Anatoly, T. S.; Alan, R. K. Heterocycles in Life and Society, Wiley, New York, 2011, pp. 209~246.
[2] Lee, E. S.; Kwon, Y. J.; Karki, R.; Jun, K.Y.; Kadayat, T. M.; Bist, G. Eur. J. Med. Chem. 2016, 113, 228.
[3] Demirci, F.; Khan, S.; Kaplancikli, Z. A. Eur. J. Med. Chem. 2010, 45, 2080.
[4] Etify, A.; Mohamed, E. A.; Aly, A.; Shaban, A. A. J. Agric. Food. Chem. 2014, 62, 9982
[5] Pae, A.N.; Baek, D. J.; Keum, G.; Nam, M.; Kim, T. H.; Seo, S. H.; Min, S. J. Eur. J. Med. Chem. 2015, 97, 245.
[6] Wang, H.-S; Liu, L.-X.; Qu, H.-E.; Huang, R.-Z.; Yao, G.-Y. Eur. J. Med. Chem. 2015, 95, 400.
[7] Chen, Y.; Wang, Z.-B.; Zhang, X.; Xia, L.-J.; Gong, H.-Y.; Zhao, H.-J.; Xue, W. Chin. J. Org. Chem. 2014, 34, 1662 (in Chinese).(陈玉, 王忠波, 张贤, 夏丽娟, 龚华玉, 赵洪菊, 薛伟, 有机化学, 2014, 34, 1662.)
[8] Dai, H.; Huang, J.-H.; Jin, Z.-C.; Cheng, X.-Y.; Huang, K.-W.; Ling, Y.; Wang, Q.-M.; Shi, Y.-J. Chin. J. Org. Chem. 2015, 35, 2617 (in Chinese).(戴红, 黄菊华, 金智超, 成晓燕, 黄凯薇, 凌勇, 汪清民, 石玉军, 有机化学, 2015, 35, 2617.)
[9] Zhou, S.-F.; Li, F.-B.; Zhang, P.-Z.; Jiang, L. Res. Chem. Intermed. 2013, 39, 1735.
[10] Wang, Q.-M.; Sun, R.-F.; Li, Y.-Q.; Xiong, L.-X. Bioorg. Med. Chem. Lett. 2010, 20, 4693.
[11] Zhang, Y.-B. World Pestic. 2013, 35, 61 (in Chinese).(张亦冰, 世界农药, 2013, 35, 61.)
[12] Liu, A.; Wang, X.; Chen, C.; Pei, H.; Mao, C.; Wang, Y.; He, H.; Huang, L.; Liu, X.; Hu, Z.; Ou, X.; Huang, M.; Yao, J. Pest. Mannge. Sci. 2009, 65, 229.
[13] Wang, M.-Y.; Qu, Z.-Q.; Du, D. Jiang, L. Chin. J. Org. Chem. 2013, 33, 1005 (in Chinese).(王美岩, 曲智强, 杜丹, 姜林, 有机化学, 2013, 33, 1005.)
[14] Zahouily, M; Mounir, B.; Cherki, H.; Bahlaouan, B.; Rayadh, A.; Sebti, S. Phosphorus, Sulfur Silicon Relat. Elem. 2007, 182, 1203.
[15] Ying, A.-G.; Zheng, R.-H.; Wu, C.-L.; Liang, H.-D.; Ge, C.-H.; Jiang, H.-D. Chin. J. Org. Chem. 2011, 31, 1312 (in Chinese).(应安国, 郑人华, 武承林, 梁华定, 葛昌华, 蒋华江, 有机化学, 2011, 31, 1312.)
[16] Zhao, Y.-X.; Sun, Y.-X. Spectral Identification for Organic Molecules, Science Press, Beijing, 2003, p. 162 (in Chinese).(赵瑶星, 孙祥玉, 有机分子结构光谱鉴定, 科学出版社, 北京, 2003, p. 162.)
[17] Ying, A.-G. Ph.D. Dissertation, Zhejiang University, Hangzhou, 2010 (in Chinese).(应安国, 博士论文, 浙江大学, 杭州, 2010.)
[18] Zakharychev, V. V.; Kuzenkov, A. V. Chem. Heterocycl. Compd. 2007, 43, 989.
[19] Li, X.-H.; Ji, M.-S.; Qi, Z.-Q.; Li, X.-W.; Shen, Y.-X.; Zhang, Y.; Wei, S.-H.; Wang, Y.-Z.; Wang, D.-Q. Pest Manage. Sci. 2011, 67, 986.
/
| 〈 |
|
〉 |