

水合肼选择性还原炔烃至顺式烯烃研究
收稿日期: 2017-01-20
修回日期: 2017-02-19
网络出版日期: 2017-02-27
基金资助
国家自然科学基金(Nos.21372059,21572044)和河北省自然科学基金(Nos.B2016201254,2016201031)资助项目.
Hydrogenation of Alkynes to cis-Alkenes with Hydrazine in Air
Received date: 2017-01-20
Revised date: 2017-02-19
Online published: 2017-02-27
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21372059, 21572044), and the Natural Science Foundation of Hebei Province (Nos. B2016201254, 2016201031).
王金涛 , 熊就凯 , 李娟娟 , 王佳利 , 王克让 , 李小六 . 水合肼选择性还原炔烃至顺式烯烃研究[J]. 有机化学, 2017 , 37(6) : 1407 -1411 . DOI: 10.6023/cjoc201701040
Transfer hydrogenation is a mild and effective way in reduction reaction. In this paper, a simple approach of selectively hydrogenation of alkynes to cis-alkenes with NH2NH2·H2O as a transfer hydrogenation agent in the presence of air without any catalysts or metals was developed. Furthermore, the configuration of cis-alkene was confirmed by the 2D NOESY spectrum.
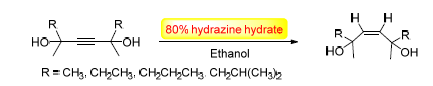
Key words: transfer hydrogenation; hydrazine; alkynes; alkenes; diazene
[1] (a) de Vries, J. G.; Elsevier, C. J. The Handbook of Homogeneous Hydrogenation. Wiley-VCH, Weinheim, 2007.
(b) Chernichenko, K.; Madarász, Á.; Pápai, I.; Nieger, M.; Leskelä, M.; Repo, T. Nat. Chem. 2013, 5, 718.
[2] (a) Johnstone, R. A. W.; Wilby, A. H.; Entwistle, I. D. Chem. Rev. 1985, 85, 129.
(b) Hauwert, P.; Maestri, G.; Sprengers, J. W.; Catellani, M.; Elsevier, C. J. Angew. Chem. Int. Ed. 2008, 47, 3223.
(c) Hauwert, P.; Boerleider, R.; Waesink, S.; Weigand, J. J.; Elsevier, C. J. J. Am. Chem. Soc. 2010, 132, 16900.
(d) Heim, L. E.; Thiel, D.; Gedig, C.; Deska, J.; Prechtl, M. H. G. Angew. Chem., Int. Ed. 2015, 54, 10308.
(e) Yang, X. H.; Xie, Z. M.; He, J.; Yu, L. Chin. J. Org. Chem. 2015, 35, 603 (in Chinese). (阳香华, 谢珍茗, 何军, 余林, 有机化学, 2015, 35, 603.)
(f) Dai, N.; Shang, R.; Fu, M. C.; Fu, Y. Chin. J. Chem. 2015, 33, 405.
[3] Wang, D.; Astruc, D. Chem. Rev. 2015, 115, 6621.
[4] (a) Hünig, S.; Müller, H. R.; Thier, W. Angew. Chem., Int. Ed. Engl. 1965, 4, 271.
(b) Pasto, D. J. Org. Rect. 1991, 40, 91.
(c) Gutmann, B.; Cantillo, D.; Kappe, C. O. Angew. Chem., Int. Ed. 2015, 54, 6688.
[5] (a) Imada, Y.; Iida, H.; Naota, T. J. Am. Chem. Soc. 2005, 127, 14544.
(b) Teichert, J. F.; den Hartog, T.; Hanstein, M.; Smit, C.; ter Horst, B.; Hernandez-Olmos, V.; Feringa, B. L.; Minnaard, A. J. ACS Catal. 2011, 1, 309.
(c) Lida, H.; Imada, Y.; Murahashi, S. I. Org. Biomol. Chem. 2015, 13, 7599.
[6] Schulz, G. A. S.; Comin, E.; de Souza, R. F. J. Appl. Polym. Sci. 2012, 123, 3605.
[7] (a) Pieber, B.; Martinez, S. T.; Cantillo, D.; Kappe, C. O. Angew. Chem., Int. Ed. 2013, 52, 10241.
(b) Santra, S.; Guin, J. Eur. J. Org. Chem. 2015, 7253.
[8] (a) Feth, M. P.; Rossen, K.; Burgard, A. Org. Process Res. Dev. 2013, 17, 282.
(b) Pieber, B.; Glasnov, T.; Kappe, C. O. Chem.-Eur. J. 2015, 21, 4368.
[9] Smit, C.; Fraaije, M. W.; Minnaard, A. J. J. Org. Chem. 2008, 73, 9482.
[10] Imada, Y.; Iida, H.; Kitagawa, T.; Naota, T. Chem.-Eur. J. 2011, 17, 5908.
[11] Lamani, M.; Ravikumara, G. S.; Prabhu, K. R. Adv. Synth. Catal. 2012, 354, 1437.
[12] Lamani, M.; Guralamata, R. S.; Prabhu, K. R. Chem. Commun. 2012, 48, 6583.
[13] Menges, N.; Balci, M. Synlett 2014, 25, 671.
[14] (a) Yu, T. B.; Bai, J. Z.; Guan, Z. Angew. Chem., Int. Ed. 2009, 48, 1097.
(b) Stockdill, J. L.; lopez, A. M.; Ibrahim, A. A. Tetrahedron Lett. 2015, 56, 3503.
(c) Hua, J.; Lam, J. W. Y.; Yu, X. M.; Wu, L. J.; Kwok, H. S.; Wong, K. S.; Tang, B. Z. J. Polym. Sci: Pol. Chem. 2008, 46, 2025.
[15] Wang, K. R.; Yang, Z. B.; Li, X. L. Chem.-Eur. J. 2015, 21, 5680.
[16] Sajiki, H.; Mori, S.; Ohkubo, T.; Ikawa, T.; Kume, A.; Maegawa, T.; Monguchi, Y. Chem.-Eur. J. 2008, 14, 5109.
/
| 〈 |
|
〉 |