

碱性离子液体中吲哚与芳基硫酚的3-芳烃硫基化反应
收稿日期: 2017-07-05
修回日期: 2017-08-16
网络出版日期: 2017-08-18
基金资助
国家自然科学基金(Nos.21502055,21642005)、中央高校基本科研业务费(No.2015ZM150)和中国博士后科学基金(No.2016T90779)资助项目.
Direct 3-Arylsulfenylation of Indoles with Thiols in Basic Ionic Liquid
Received date: 2017-07-05
Revised date: 2017-08-16
Online published: 2017-08-18
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21502055, 21642005), the Fundamental Research Funds for the Central Universities (No. 2015ZM150) and the China Postdoctoral Science Foundation (No. 2016T90779).
岑竞鹤 , 杨凯 , 李建晓 , 李灿 , 杨少容 . 碱性离子液体中吲哚与芳基硫酚的3-芳烃硫基化反应[J]. 有机化学, 2017 , 37(12) : 3213 -3219 . DOI: 10.6023/cjoc201707005
An efficient and convenient transition metal-free procedure for the synthesis of 3-sulfenylindoles derivatives in moderate to good yields from readily available indoles and thiols in basic ionic liquid has been developed. Their structures were confirmed by 1H NMR, 13C NMR and HRMS. This sulfenylation process provides a novel route for directly accessing 3-sulfenylindoles in good to excellent yields and good functional group tolerance with high atom efficiency. Notably, the current methodology could also be conveniently applied to the synthesis of thioarylated naturally occurring biologically active frameworks.
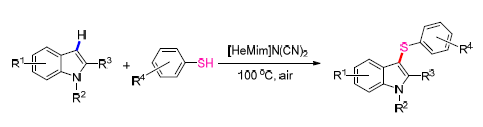
Key words: ionic liquids; indoles; thiols; 3-arylsulfenylation reaction
[1] (a) Gulevich, A. V.; Dudnik, A. S.; Chernyak, N.; Gevorgyan, V. Chem. Rev. 2013, 113, 3084.
(b) Pan, X.; Xia, H.; Wu, J. Org. Chem. Front. 2016, 3, 1163.
[2] (a) Metzner, P.; Thuillier, A.; Katritzky, A. R.; Rees, C. W. Sulfur Reagents in Organic Synthesis, Academic Press, London, 1994.
(b) Wirth, T. Organoselenium Chemistry:Modern Developments in Organic Synthesis, Springer Verlag, Berlin, NY, 2000.
(c) Zhang, B.; Kang, Y.; Shi, R. Chin. J. Org. Chem. 2016, 36, 1814(in Chinese). (张变香, 亢永强, 史瑞雪, 有机化学, 2016, 36, 1814.)
[3] Bierbeek, A. V.; Gingras, M. Tetrahedron Lett. 1998, 39, 6283.
[4] (a) Qiao, Z.; Jiang, X. Org. Lett. 2016‚ 18‚ 1550.
(b) Jiang, Y.; Liang, G.; Zhang, C.; Loh, T.-P. Eur. J. Org. Chem. 2016, 3326.
[5] (a) Taniguchi, N. J. Org. Chem. 2004, 69, 6904.
(b) Wang, Y.; Zhang, X.; Liu, H.; Chen, H.; Huang, D. Org. Chem. Front. 2017, 4, 31.
[6] (a) Ravi, C.; Reddy, N. N. K.; Venkatanarayana, P.; Samanta, S.; Adimurthy, S. J. Org. Chem. 2016, 81, 9964.
(b) Matheis, C.; Bayarmagnai, B.; Goossen, L. J. Org. Chem. Front. 2016, 3, 949.
(c) Wen, L.-R.; Shen, Q.-Y.; Guo, W.-S.; Li, M. Org. Chem. Front. 2016, 3, 870.
[7] (a) Correa, A.; Carril, M.; Bolm, C. Angew. Chem., Int. Ed. 2008, 47, 2880.
(b) Fu, R.; Hao, W.-J.; Wu, Y.-N.; Wang, N.-N.; Tu, S.-J.; Li, G.; Jiang, B. Org. Chem. Front. 2016, 3, 1452.
[8] (a) Reddy, V. P.; Swapna, K.; Kumar, A. V.; Rao, K. R. J. Org. Chem. 2009, 74, 3189.
(b) Xi, Y.; Dong, B.; McClain, E. J.; Wang, Q.; Gregg, T. L.; Akhmedov, N. G.; Petersen, J. L.; Shi, X. Angew. Chem., Int. Ed. 2014, 53, 4657.
(c) Xiong, H.-Y.; Pannecoucke, X.; Besset, T. Org. Chem. Front. 2016, 3, 620.
[9] (a) Yang, F.-L.; Tian, S.-K. Angew. Chem., Int. Ed. 2013, 52, 4929.
(b) Yuan, J.; Liu, C.; Lei, A. Org. Chem. Front. 2015, 2, 677.
(c) Yang, F.-L.; Gui, Y.; Yu, B.-K.; Jin, Y-X.; Tian, S.-K. Adv. Synth. Catal. 2016, 358, 3368.
[10] Funk, C. D. Nat. Rev. Drug Discovery 2005, 4, 664.
[11] (a) Ge, W.; Wei, Y. Green Chem. 2012, 14, 2066.
(b) Azeredo, J. B.; Godoi, M.; Martins, G. M.; Silveira, C. C.; Braga, A. L. J. Org. Chem. 2014, 79, 4125.
(c) Xiao, F.; Xie, H.; Liu, S.; Deng, G.-J. Adv. Synth. Catal. 2014, 356, 364.
[12] (a) Zou, L.-H.; Reball, J.; Mottweiler, J.; Bolm, C. Chem. Commun. 2012, 48, 11307.
(b) Gao, Z.; Zhu, X.; Zhang, R. RSC Adv. 2014, 4, 19891.
[13] (a) Prasad, C. D.; Balkrishna, S. J.; Kumar, A.; Bhakuni, B. S.; Shrimali, K.; Biswas, S.; Kumar, S. J. Org. Chem. 2013, 78, 1434.
(b) Rao, H.; Wang, P.; Wang, J.; Li, Z.; Sun, X.; Cao, S. RSC Adv. 2014, 4, 49165.
[14] (a) Li, X.; Xu, Y.; Wu, W.; Jiang, C.; Qi, C.; Jiang, H. Chem.-Eur. J. 2014, 20, 7911.
(b) Xu, Y.; Tang, X.; Hu, W.; Wu, W.; Jiang, H. Green Chem. 2014, 16, 3720.
(c) Gao, Y.; Gao, Y.; Tang, X.; Peng, J.; Hu, M.; Wu, W.; Jiang, H. Org. Lett. 2016, 18, 1158.
[15] (a) Li, J.; Yang, S.; Jiang, H.; Wu, W.; Zhao, J. J. Org. Chem. 2013, 78, 12477.
(b) Li, J.; Yang, S.; Huang, L.; Chen, H.; Jiang, H. RSC Adv. 2013, 3, 11529.
(c) Li, J.; Yang, S.; Wu, W.; Jiang, H. Chem. Commun. 2014, 50, 1381.
(d) Li, J.; Zhu, Z.; Yang, S.; Zhang, Z.; Wu, W.; Jiang, H. J. Org. Chem. 2015, 80, 3870.
(e) An, Y.; Li, J.; Zhang, Z.; Li, C.; Yang, S. Chin. J. Org. Chem. 2016, 36, 2136(in Chinese). (安艳妮, 李建晓, 张振明, 李春生, 杨少容, 有机化学, 2016, 36, 2136.)
(f) An, Y.; Li, J.; Li, M.; Li, C.; Yang, S. Chin. J. Org. Chem. 2017, 37, 720(in Chinese). (安艳妮, 李建晓, 李蒙, 李春生, 杨少容, 有机化学, 2017, 37, 720.)
(g) Li, J.; An, Y.; Li, J.; Yang, S.; Wu, W.; Jiang, H. Org. Chem. Front. 2017, 4, 1590.
[16] (a) Li, J.; Li, C.; Yang, S.; An, Y.; Wu, W.; Jiang, H. J. Org. Chem. 2016, 81, 2875.
(b) Li, J.; Li, C.; Yang, S.; An, Y.; Wu, W.; Jiang, H. J. Org. Chem. 2016, 81, 7771.
(c) Li, J.; Hu, W.; Li, C.; Yang, S.; Wu, W.; Jiang, H. Org. Chem. Front. 2017, 4, 373.
[17] Dou, H.; Gao, S.; Fu, Z.; Liu, S. Chin. J. Org. Chem. 2011, 31, 1056(in Chinese). (窦辉, 高思旖, 付召龙, 刘士忠, 有机化学, 2011, 31, 1056.)
[18] Sang, P.; Chen, Z.; Zou, J.; Zhang, Y. Green Chem. 2013, 15, 2096.
[19] Huang, D.; Chen, J.; Dan, W.; Ding, J.; Liu, M.; Wu, H. Adv. Synth. Catal. 2012, 354, 2123.
[20] Tudge, M.; Tamiya, M.; Savarin, C.; Humphrey, G. R. Org. Lett. 2006, 8, 565.
[21] Guo, Y.-J.; Tang, R.-Y.; Li, J.-H.; Zhong, P.; Zhang, X.-G. Adv. Synth. Catal. 2009, 351, 2615.
/
| 〈 |
|
〉 |