

1,8-二氮杂二环十一碳-7-烯(DBU)立体选择性催化合成噁唑啉-2-(硫)酮衍生物
收稿日期: 2017-04-21
修回日期: 2017-08-10
网络出版日期: 2017-09-08
基金资助
国家自然科学基金(No.21602123)及三峡大学青年拔尖人才项目和研究生创新基金(No.SDYC2016121)资助项目.
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU)-Promoted Stere-oselective Synthesis of Oxazolidin-2-(thi)one Derivatives
Received date: 2017-04-21
Revised date: 2017-08-10
Online published: 2017-09-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21602123), the Youth Talent Development Foundation and Scientific Foundation from Graduate School of China Three Gorges University (No. SDYC2016121).
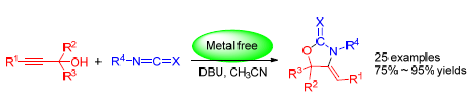
以1,8-二氮杂二环十一碳-7-烯(DBU)为催化剂,在无其它金属催化条件下,炔丙醇与异氰酸酯(或硫氰酸酯)进行亲核加成/环化反应,合成了一系列多取代的噁唑烷-2-(硫)酮衍生物,收率为75%~95%.该反应经5-exo-dig环化机制,高度立体选择性地得到Z-式芳亚甲基取代产物,为噁唑啉酮的骨架合成提供了简单快速的合成方法.
关键词: 噁唑啉-2-(硫)酮; 1,8-二氮杂二环十一碳-7-烯; 立体选择性
杨权力 , 宋玉叶 , 余平 , 王龙 , 刘明国 , 黄年玉 . 1,8-二氮杂二环十一碳-7-烯(DBU)立体选择性催化合成噁唑啉-2-(硫)酮衍生物[J]. 有机化学, 2017 , 37(12) : 3177 -3185 . DOI: 10.6023/cjoc201704036
A series of multi-substituted oxazolidin-2-(thi)one derivatives were prepared via 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) promoted nucleophilic addition/cyclization from alkynyl alcohol and isocynate or thiocynate in high yields of 75%~95%. This concise and efficient approach provides a facial access to a library of biological (Z)-arylmethylene substituted oxazolidin-2-(thi)one derivatives with high stereo-selectivities.

[1] Zappia, G.; Menendez, P.; Monache, G. D.; Misiti, D.; Nevola, L.; Botta, B. Mini-Rev. Med. Chem. 2007, 7, 389.
[2] (a) Phillips, O. A.; Sharaf, L. H. Expert Opin. Ther. Pat. 2016, 26, 591.
(b) Jadhavar, P. S.; Vaja, M. D.; Dhameliya, T. M.; Chakraborti, A. K. Curr. Med. Chem. 2015, 22, 4379.
[3] (a) Fujita, T.; Yamago, S. Chem.-Eur. J. 2015, 21, 18547.
(b) Green, R.; Peed, J.; Taylor, J. E.; Blackburn, R. A.; Bull, S. D. Nat. Protoc. 2013, 8, 1890.
[4] Ferreira, J.; Rees-Jones, S. C.; Ramaotsoa, V.; Msutu, A.; Hunter, R. Org. Biomol. Chem. 2016, 14, 1545.
[5] (a) Guo, B.; Fan, H.; Xin, Q.; Chu, W.; Wang, H.; Huang, Y.; Chen, X.; Yang, Y. J. Med. Chem. 2013, 56, 2642.
(b) Friggeri, L.; Ballante, F.; Ragno, R.; Musmuca, I.; De Vita, D.; Manetti, F.; Biava, M.; Scipione, L.; Di Santo, R.; Costi, R.; Feroci, M.; Tortorella, S. J. Chem. Inf. Model. 2013, 53, 1463.
[6] (a) Njiojob, C. N.; Bozell, J. J.; Long, B. K.; Elder, T.; Key, R. E.; Hartwig, W. T. Chem.-Eur. J. 2016, 22, 12506.
(b) Mydock-McGrane, L.; Rath, N. P.; Covey, D. F. J. Org. Chem. 2014, 79, 5636.
[7] Mendes, R. E.; Deshpande, L. M.; Jones, R. N. Drug Resist. Updates 2014, 17, 1.
[8] Chahine, E. B.; Sucher, A. J.; Knutsen, S. D. Consult. Pharm. 2015, 30, 386.
[9] (a) Xu, J. X.; Zhao, J. W.; Jia, Z. B. Chin. Chem. Lett. 2011, 22, 1063.
(b) Li, Y. W.; Liu, Y.; Jia, Y. C.; Yuan, J. Y. Chin. Chem. Lett. 2013, 24, 230.
[10] (a) Sadeghzadeh, S. M. Appl. Organomet. Chem. 2016, 30, 835.
(b) Bacchi, A.; Chiusoli, G. P.; Costa, M.; Gabriele, B.; Righi, C.; Salerno, G. Chem. Commun. 1997, 1209.
(c) Song, Q. W.; Zhou, Z. H.; Wang, M. Y.; Zhang, K.; Liu, P.; Xun, J. Y.; He, L. N. ChemSusChem 2016, 9, 2054.
(d) Hu, J.; Ma, J.; Zhu, Q.; Zhang, Z.; Wu, C.; Han, B. Angew. Chem., Int. Ed. 2015, 54, 5399.
(e) Hase, S.; Kayaki, Y.; Ikariya, T. ACS Catal. 2015, 5, 5135.
[11] (a) Fujisaki, F.; Abe, N.; Sumoto, K. Heterocycles 2008, 75, 1681.
(b) Steiner, B.; Langer, V.; Koóš, M. Carbohydr. Res. 2009, 344, 2079.
[12] (a) Li, S. Q.; Xiong, P.; Zhu, L.; Qian, X. Y.; Xu, H. C. Eur. J. Org. Chem. 2016, 2016, 3449.
(b) Sekine, K.; Mawatari, T.; Yamada, T. Synlett 2015, 26, 2447.
(c) Kim, W. S.; Yoon, E.; Jo, K. A.; Kang, E. J. Bull. Korean Chem. Soc. 2011, 32, 3158.
[13] (a) Lu, X. F.; Yang, Z.; Huang, N. Y.; He, H. B.; Deng, W. Q.; Zou, K. Bioorg. Med. Chem. Lett. 2015, 25, 3726.
(b) Wang, L.; Xie, Y. B.; Huang, N. Y.; Yan, J. Y.; Hu, W. M.; Liu, M. G.; Ding, M. W. ACS Catal. 2016, 6, 4010.
(c) Li, R. K.; Yang, Q. L.; Liu, Y.; Li, D. W.; Huang, N. Y.; Liu, M. G. Chin. Chem. Lett. 2016, 27, 345.
[14] Li, S.; Ye, J.; Yuan, W.; Ma, S. Tetrahedron 2013, 69, 10450.
[15] Zhao, J.; Huang, H.; Qi, C.; Jiang, H. Eur. J. Org. Chem. 2012, 29, 5665.
[16] (a) Ritter, S.; Horino, Y.; Lex, J.; Schmalz, H. G. Synlett 2006, 3309.
(b) Ramesh, R.; Chandrasekaran, Y.; Megha, R.; Chandrasekaran, S. Tetrahedron 2007, 63, 9153.
(c) Hu, M.; Song, R. J.; Li, J. H. Angew. Chem., Int. Ed. 2015, 54, 608.
[17] (a) Doherty, S.; Knight, J. G.; Perry, D. O.; Ward, N. A.; Bittner, D. M.; McFarlane, W.; Probert, M. R. Organometallics 2016, 35, 1265.
(b) Wang, F.; Wang, Y.; Cai, L.; Miao, Z.; Chen, R. Adv. Synth. Catal. 2008, 350, 2733.
(c) Hu, Y.; Xin, X.; Wan, B. Tetrahedron Lett. 2014, 55, 32.
[18] (a) Hashmi, A.; Stephen K.; Wang, T.; Shi, S.; Rudolph, M. J. Org. Chem. 2012, 77, 7761.
(b) Engel, D. A.; Dudley, G. B. Org. Lett. 2006, 8, 4027.
(c) Jagtap, S. R.; Bhanage, B. M. J. Chem. Res. 2007, 6, 370.
(d) Smissman, E. E.; Johnsen, R. H.; Carlson, A. W.; Aycock, B. F. J. Org. Chem. 1956, 78, 3395.
(e) Cooper, M. A.; Lucas, M. A.; Taylor, J. M.; Ward, A. D.; Williamson, N. M. Synthesis 2001, 621.
(f) Moran, W. J.; Rodríguez, A. Org. Biomol. Chem. 2012, 10, 8590.
(g) Zhang, H.; Tanimoto, H.; Morimoto, T.; Nishiyama, Y.; Kakiuchi, K. Org. Lett. 2013, 20, 5222.
/
| 〈 |
|
〉 |