

不对称串联[1,n]-氢迁移/环化反应构建手性化合物研究进展
收稿日期: 2017-08-13
修回日期: 2017-09-19
网络出版日期: 2017-10-16
基金资助
荆楚理工学院药物合成与优化湖北省重点实验室开放基金(No.OPP2015ZD02)及青岛农业大学高层次人才启动基金(No.6631115015)资助项目.
Construction of Chiral Cyclic Compounds via Asymmetric Cascade[1,n]-Hydride Transfer/Cyclization
Received date: 2017-08-13
Revised date: 2017-09-19
Online published: 2017-10-16
Supported by
Project supported by the Open Project Program of Hubei Key Laboratory of Drug Synthesis and Optimization Jingchu University of Technology (No. OPP2015ZD02) and the Talents of High Level Scientific Research Foundation of Qingdao Agricultural University (No. 6631115015).
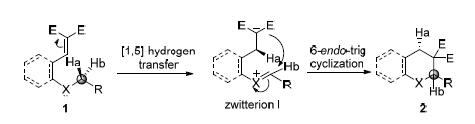
串联[1,n]-氢迁移/环化反应通过分子内氢负离子迁移,能够使杂原子邻位C(sp3)—H键官能化,把C(sp3)—H键直接转化成为C—C,C—N,C—O等键.此方法在构建五元、六元、七元杂环和全碳环中表现出了巨大的潜力,通过该反应可以高效地合成药物分子中的常见骨架.手性胺、手性路易斯酸以及手性布朗斯特酸等催化剂已经成功地应用于这类反应的不对称催化当中.
关键词: C(sp3)-H键官能化; 串联反应; [1,n]-氢迁移/环化反应; 不对称催化; 杂环化合物
肖明艳 , 朱帅 , 沈耀滨 , 王亮 , 肖建 . 不对称串联[1,n]-氢迁移/环化反应构建手性化合物研究进展[J]. 有机化学, 2018 , 38(2) : 328 -340 . DOI: 10.6023/cjoc201708024
The C (sp3)-H adjacent to heteroatoms can be readily functionalized to C-C, C-N, C-O bonds etc. via cascade[1, n]-hydride transfer/cyclization, which shows high potency to construct 5-membered, 6-membered and all carbon rings. This intriguing cascade process can be employed to synthesize common skeletons of significant natural products and pharmaceutical molecules. Chiral amines, Lewis acids and Brønsted acids have been successfully utilized to catalyze the asymmetric cascade reaction.

[1] (a) Mkhalid, I. A. I.; Barnard, J. H.; Marder, T. B.; Murphy, J. M.; Hartwig, J. F. Chem. Rev. 2010, 110, 890.
(b) Giri, R.; Shi, B. F.; Engle, K. M.; Maugel, N.; Yu, J. Q. Chem. Soc. Rev. 2009, 38, 3242.
(c) He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J. -Q. Chem. Rev. 2017, 117, 8754;(d) Davies, H. M.; Du Bois, J.; Yu, J. Q. Chem. Soc. Rev. 2011, 40, 1855.
(e)Yang, J.; Fu, T.; Long, Y.; Zhou, X. Chin. J. Org. Chem. 2017, 37, 1111(in Chinese). (杨军, 付婷, 龙洋, 周向葛, 有机化学, 2017, 37, 1111.)
[2] (a) Girard, S. A.; Knauber, T.; Li, C. J. Angew. Chem., Int. Ed. 2014, 53, 74.
(b) Li, C. -J. Acc. Chem. Res. 2009, 42, 335.
[3] (a) DiRocco, D. A.; Rovis, T. J. Am. Chem. Soc. 2012, 134, 8094.
(b) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. Chem. Rev. 2013, 113, 5322.
[4] Fu, N.; Li, L.; Yang, Q.; Luo, S. Org. Lett. 2017, 19, 2122.
[5] Campos, K. R. Chem. Soc. Rev. 2007, 36, 1069.
[6] (a) Zhang, S.; Zhang, F.; Tu, Y. Chem. Soc. Rev. 2011, 40, 1937.
(b) Guo, S.; Kumar, P. S.; Yang, M. Adv. Synth. Catal. 2017, 359, 2.
(c) Zhang, J. -R.; Xu, L.; Liao, Y. -Y.; Deng, J. -C.; Tang, R. -Y. Chin. J. Chem. 2017, 35, 271.
(d) Shang, X.; Liu, Z. Acta Chim. Sinica 2015, 73, 1275(in Chinese). (尚筱洁, 柳忠全, 化学学报, 2015, 73, 1275.)(e) Liu, L.; Floreancig, P. E. Angew. Chem., Int. Ed. 2010, 49, 5894.
(f) Tu, W.; Floreancig, P. E. Angew. Chem., Int. Ed. 2009, 48, 4567.
(g) Tu, W.; Liu, L.; Floreancig, P. E. Angew. Chem., Int. Ed. 2008, 47, 4184.
[7] (a) Haibach, M. C.; Seidel, D. Angew. Chem., Int. Ed. 2014, 53, 5010.
(b) Peng, B.; Maulide, N. Chem. -Eur. J. 2013, 19, 13274.
(c)Wang, L.; Xiao, J. Adv. Synth. Catal. 2014, 356, 1137.
(d)Wang, L.; Xiao, J. Top. Curr. Chem. 2016, 374, 17.
[8] Pinnow, J. Ber. Dtsch. Chem. Ges. 1895, 28, 3039.
[9] (a) Alajarin, M.; Bonillo, B.; Marin-Luna, M.; Sanchez-Andrada, P.; Vidal, A. Chem. -Eur. J. 2013, 47, 16093.
(b) Zhao, S.; Shu, X.; Ji, K.; Zhou, A.; He, T.; Liu, X.; Liang, Y. J. Org. Chem. 2011, 76, 1941.
(c) Alajarin, M.; Bonillo, B.; Ortin, M. -M.; Sanchez-Andrada, P.; Vidal, A. Eur. J. Org. Chem. 2011, 1896.
(d)Alajarin, M.; Bonillo, B.; Ortin, M.; Sanchez-Andrada, P.; Vidal, A.; Orenes, R. Org. Biomol. Chem. 2010, 8, 4690.
(e)Alajarin, M.; Bonillo, B.; Orenes, R. A.; Ortin, M. M.; Vidal, A. Org. Biomol. Chem. 2012, 10, 9523.
[10] (a) Ruble, J. C.; Hurd, A. R.; Johnson, T. A.; Sherry, D. A.; Barbachyn, M. R.; Toogood, P. L.; Bundy, G. L.; Graber, D. R.; Kamilar, G. M. J. Am. Chem. Soc. 2009, 131, 3991.
(b) Woelfling, J.; Frank, É.; Schneider, G.; Tietze, L. Angew. Chem., Int. Ed. 1999, 38, 200.
(c) Wölfling, J.; Frank, E.; Schneider, G.; Tietze, L. F. Eur. J. Org. Chem. 1999, 3013.
(d) Wölfling, J.; Frank, E.; Schneider, G.; Tietze, L. F. Eur. J. Org. Chem. 2004, 90.
[11] Murarka, S.; Zhang, C.; Konieczynska, M. D.; Seidel, D. Org. Lett. 2009, 11, 129.
[12] (a) Mahlau, M.; List, B. Angew. Chem., Int. Ed. 2013, 52, 518.
(b) Du, Y.; Luo, S.; Gong, L. Tetrahedron Lett. 2011, 52, 7064.
[13] Mori, K.; Ehara, K.; Kurihara, K.; Akiyama, T. J. Am. Chem. Soc. 2011, 133, 6166.
[14] Cao, W.; Liu, X.; Wang, W.; Lin, L.; Feng, X. Org. Lett. 2011, 13, 600.
[15] (a) Chen, L.; Zhang, L.; Lv, J.; Cheng, J.; Luo, S. Chem. -Eur. J. 2012, 18, 8891.
(b) Zhang, L.; Chen, L.; Lv, J.; Cheng, J. -P.; Luo, S. Chem. -Asian J. 2012, 7, 2569.
[16] Murarka, S.; Deb, I.; Zhang, C.; Seidel, D. J. Am. Chem. Soc. 2009, 131, 13226.
[17] Han, Y.; Han, W.; Hou, X.; Zhang, X.; Yuan, W. Org. Lett. 2012, 14, 4054.
[18] Cao, W.; Liu, X.; Guo, J.; Lin, L.; Feng, X. Chem. -Eur. J. 2015, 21, 1632.
[19] Lin, X.; Mao, Z.; Mo, F. Synlett 2015, 27, 546.
[20] Kang, Y. K.; Kim, S. M.; Kim, D. Y. J. Am. Chem. Soc. 2010, 132, 11847.
[21] Suh, C. W.; Kim, D. Y. Org. Lett. 2014, 16, 5374.
[22] Suh, C. W.; Woo, S. B.; Kim, D. Y. Asian J. Org. Chem. 2014, 3, 399.
[23] Kang, Y. K.; Kim, D. Y. Chem. Commun. 2014, 50, 222.
[24] Kang, Y. K.; Kim, D. Y. Adv. Synth. Catal. 2013, 355, 3131.
[25] He, Y. P.; Wu, H.; Chen, D. F.; Yu, J.; Gong, L. Z. Chem. -Eur. J. 2013, 19, 5232.
[26] Suh, C. W.; Kwon, S. J.; Kim, D. Y. Org. Lett. 2017, 19, 1334.
[27] (a) Zhou, G.; Liu, F.; Zhang, J. Chem. -Eur. J. 2011, 17, 3101.
(b) Zhou, G.; Zhang, J. Chem. Commun. 2010, 46, 6593.
[28] Wang, P. F.; Jiang, C. H.; Wen, X.; Xu, Q. L.; Sun, H. J. Org. Chem. 2015, 80, 1155.
[29] Jiao, Z.; Zhang, S.; He, C.; Tu, Y.; Wang, S.; Zhang, F.; Zhang, Y.; Li, H. Angew. Chem., Int. Ed. 2012, 51, 8811.
[30] Liao, S.; Sun, X. L.; Tang, Y. Acc. Chem. Res. 2014, 47, 2260.
[31] Frank, É.; Schneider, G.; Kádár, Z.; Wölfling, J. Eur. J. Org. Chem. 2009, 3544.
/
| 〈 |
|
〉 |