

氨基酸多相催化转化研究进展
收稿日期: 2017-09-04
修回日期: 2017-10-12
网络出版日期: 2017-11-03
基金资助
浙江省自然科学基金(No.LY18B020018)资助项目.
Progress on the Transformations of Amino Acids by Heterogeneous Catalysis
Received date: 2017-09-04
Revised date: 2017-10-12
Online published: 2017-11-03
Supported by
Project supported by the Natural Science Foundation of Zhejiang Province (No. LY18B020018).
王胜 , 许孝良 , 李小年 . 氨基酸多相催化转化研究进展[J]. 有机化学, 2018 , 38(3) : 565 -574 . DOI: 10.6023/cjoc201709003
The catalytic transformation of amino acids is one of the important routes in utilization of amino acids in chemical and biological fields. In this review, catalytic hydrogenation from amino acids to chiral amino alcohols, catalytic decarboxylation to produce amine and nitrile, catalytic deamination to produce carboxylic acid and its derivatives, catalytic pyrolysis to produce bio-fuel and the application as heterogeneous chiral catalyst were summarized. In the catalytic hydrogenation of amino acids, Ru and Rh-based catalysts showed better catalytic performance, and the temperature was a main factor on the optical purity of the product. The decarboxylation, deamination and pyrolysis reaction required relatively high temperature, which needed a large amount of energy consumption. The search of high activity and selectivity heterogeneous catalyst to achieve the reduction of the reaction temperature and pressure is the focus of future research. As the heterogeneous chiral catalyst, the research should be focus on the efficiency, seperation and recycling of the catalyst.
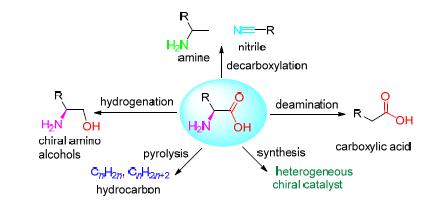
Key words: amino acid; heterogeneous catalysis; catalytic transformation
[1] (a) Corma, A.; Iborra, S.; Velty, A. Chem. Rev. 2007, 107, 2411.
(b) Sheldon, R. A. Green Chem. 2014, 16, 950.
(c) Gilkey, M. J.; Xu, B. ACS Catal. 2016, 6, 1420.
[2] Tuck, C. O.; Pérez, E.; Horváth, I. T.; Sheldon, R. A.; Poliakoff, M. Science 2012, 337, 695.
[3] Breuer, M.; Ditrich, K.; Habicher, T.; Hauer, B.; Kesseler, M.; Stürmer, R.; Zelinski, T. Angew. Chem., Int. Ed. 2004, 43, 788.
[4] Demain, A. L. Ind. Biotech. 2007, 3, 269.
[5] Corey, E. J.; Bakshi, R. K.; Shibata, S. J. Am. Chem. Soc. 1987, 109, 5551.
[6] Rogers, G. A.; Parsons, S. M.; Anderson, D. C.; Nilsson, L. M.; Bahr, B. A.; Kornreich, W. D.; Kaufman, R.; Jacobs, R. S.; Kirtman, B. J. Med. Chem. 1989, 32, 1217.
[7] Corey, E. J.; Zhang, F. Y. Angew. Chem., Int. Ed. 1999, 38, 1931.
[8] (a) Abdelrahman, O. A.; Heyden, A.; Bond, J. Q. ACS Catal. 2014, 4, 1171.
(b) Tan, J.; Cui, J.; Cui, X.; Deng, T.; Li, X.; Zhu, Y.; Li, Y. ACS Catal. 2015, 5, 7379.
(c) Zhou, M.; Zhang, H.; Ma, H.; Ying, W. Ind. Eng. Chem. Res. 2017, 56, 8833.
[9] (a) Wang, F.; Zhang, Z. ACS Sustainable Chem. Eng. 2016, 5, 942.
(b) Adkins, H.; Pavlic, A. A. J. Am. Chem. Soc. 1947, 69, 3039.
(c) Zhu, Y.; Zhu, Y.; Ding, G.; Zhu, S.; Zheng, H.; Li, Y. Appl. Catal., A 2013, 468, 296.
(d) Zheng, X.; Lin, H.; Zheng, J.; Duan, X.; Yuan, Y. ACS Catal. 2013, 3, 2738.
[10] Di, X.; Li, C.; Zhang, B.; Qi, J.; Li, W.; Su, D.; Liang, C. Ind. Eng. Chem. Res. 2017, 56, 4672.
[11] Primo, A.; Concepción, P.; Corma, A. Chem. Commun. 2011, 47, 3613.
[12] Fan, G.; Zhou, Y.; Fu, H.; Ye, X.; Li, R.; Chen, H.; Li, X. Chin. J. Chem. 2011, 29, 229.
[13] Adkins, H., Billica, H. R. J. Am. Chem. Soc. 1948, 70, 3121.
[14] (a) Antons, S.; Beitzke, B. DE 4428106, 1996[Chem. Abstr. 1996, 124, 288759].
(b) Antons, S. DE 4444109, 1996[Chem. Abstr. 1996, 125, 114175].
[15] Antons, S.; Tilling, A. S.; Wolters, E. WO 9938838, 1999[Chem. Abstr. 1999, 131, 130283].
[16] Mägerlein, W.; Dreisbach, C.; Hugl, H.; Tse, M. K.; Klawonn, M.; Bhor, S.; Beller, M. Catal. Today 2007, 121, 140.
[17] Metkar, P. S.; Scialdone, M. A.; Moloy, K. G. Green Chem. 2014, 16, 4575.
[18] Gong, D.-C.; Tu, Z.-Y.; He, H.-H.; Wei, P.; Ou Yang, P.-K. Mod. Chem. Ind. 2007, 27, 151(in Chinese). (龚大春, 涂志英, 何红华, 韦萍, 欧阳平凯, 现代化工, 2007, 27, 151.)
[19] Tamura, M.; Tamura, R.; Takeda, Y.; Nakagawa, Y.; Tomishige, K. Chem. Commun. 2014, 50, 6656.
[20] Jere, F. T. Ph.D. Dissertation, Michigan State University, East Lansing, 2003.
[21] Tamura, M.; Tamura, R.; Takeda, Y.; Nakagawa, Y.; Tomishige, K. Chem.-Eur. J. 2015, 21, 3097.
[22] Holladay, J. E.; Werpy, T. A.; Muzatko, D. S. In Proceedings of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals Held May 4~7, Breckenridge, Humana Press, Clifton, 2003, pp. 857~869.
[23] Jere, F. T.; Miller, D. J.; Jackson, J. E. Org. Lett. 2003, 5, 527.
[24] Wang, Y. M.S. Thesis, Tianjin University, Tianjin, 2007(in Chinese). (王毅, 硕士论文, 天津大学, 天津, 2007.)
[25] He, H.-H. M.S. Thesis, Nanjing Tech University, Nanjing, 2005(in Chinese). (何洪华, 硕士论文, 南京工业大学, 南京, 2005.)
[26] Zwietering, T. N. Chem. Eng. Sci. 1958, 8, 244.
[27] Jere, F. T.; Jackson, J. E.; Miller, D. J. Ind. Eng. Chem. Res. 2004, 43, 3297.
[28] Bhandare, S. G.; Vaidya, P. D. Ind. Eng. Chem. Res. 2017, 56, 3797.
[29] Pimparkar, K. P.; Miller, D. J.; Jackson, J. E. Ind. Eng. Chem. Res. 2008, 47, 7648.
[30] Roose, P.; Eller, K.; Henkes, E.; Rossbacher, R.; Höke, H. Amines, Aliphatic in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag, Weinheim, Germany, 2015, pp. 1~55.
[31] Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J. P.; Boutevin, B. Chem. Rev. 2016, 116, 14181.
[32] De Schouwer, F.; Claes, L.; Claes, N.; Bals, S.; Degrève, J.; De Vos, D. E. Green Chem. 2015, 17, 2263.
[33] Verduyckt, J.; Van Hoof, M.; De Schouwer, F.; Wolberg, M.; Kurttepeli, M.; Eloy, P.; Gaigneaux, E. M.; Bals, S.; Kirschhock, C. E. A.; De Vos, D. E. ACS Catal. 2016, 6, 7303.
[34] Verduyckt, J.; Coeck, R.; De Vos, D. E. ACS Sustainable Chem. Eng. 2017, 5, 3290.
[35] Claes, L.; Verduyckt, J.; Stassen, I.; Lagrain, B.; De Vos, D. E. Chem. Commun. 2015, 51, 6528.
[36] Claes, L.; Matthessen, R.; Rombouts, I.; Stassen, I.; De Baerdemaeker, T.; Depla, D.; Delcour, J. A.; Lagrain, B.; DeVos, D. E. ChemSusChem 2015, 8, 345.
[37] De Schouwer, F.; Cuypers, T.; Claes, L.; De Vos, D. E. Green Chem. 2017, 19, 1866.
[38] Liu, G.; Wright, M. M.; Zhao, Q.; Brown, R. C.; Wang, K.; Xue, Y. Energy Convers. Manage. 2016, 112, 220.
[39] Yi, L.; Liu, H.; Lu, G.; Zhang, Q.; Wang, J.; Hu, H.; Yao, H. Energ. Fuel. 2017, 31, 9484.
[40] Eder, U.; Sauer, G.; Wiechert, R. Angew. Chem., Int. Ed. 1971, 10, 496.
[41] List, B.; Lerner, R. A.; Barbas, C. F. J. Am. Chem. Soc. 2000, 122, 2395.
[42] List, B. Tetrahedron 2002, 58, 5573.
[43] (a) Wang, J.-Z. M.S. Thesis, Beijing University of Chemical Technology, Beijing, 2011(in Chinese). (王玖钊, 硕士论文, 北京化工大学, 北京, 2011.)
(b) Gruttadauria, M.; Giacalone, F.; Noto, R. Adv. Synth. Catal. 2009, 351, 33.
(c) Doyagüez, E. G.; Calderon, F.; Sanchez, F.; Fernandez-Mayoralas, A. J. Org. Chem. 2007, 72, 9353.
[44] Gao, J.; Liu, J.; Jiang, D.; Xiao, B.; Yang, Q. J. Mol. Catal. A:Chem. 2009, 313, 79.
[45] An, Z.; Zhang, W.; Shi, H.; He, J. J. Catal. 2006, 241, 319.
[46] An, Z.; Guo, Y.; Zhao, L.; Li, Z.; He, J. ACS Catal. 2014, 4, 2566.
/
| 〈 |
|
〉 |