

通过金属有机小分子水凝胶选择性形成实现磷酸根的可视识别
收稿日期: 2017-09-29
修回日期: 2017-11-11
网络出版日期: 2017-11-21
基金资助
国家重点研发项目(No.2016YFA0202902)及国家自然科学基金(No.21572036)资助项目.
Visual Discrimination of Phosphate Anion via Selective Metallo-Hydrogel Formation
Received date: 2017-09-29
Revised date: 2017-11-11
Online published: 2017-11-21
Supported by
Project supported by the National Key R&D Program of China (No. 2016YFA0202902) and the National Natural Science Foundation of China (No. 21572036).
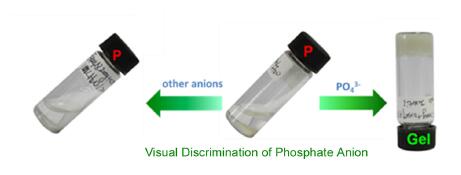
利用磷酸根和钳型三联吡啶锌络合物的金属中心锌离子具有选择性配位的能力,通过金属有机水凝胶的选择性形成实现了对磷酸根的可视识别.在此基础上,利用一系列实验对这类金属有机水凝胶的热稳定性、流变性质、外观样貌进行了详细表征和分析之后提出了对磷酸根选择性识别的可能机理.
关键词: 金属有机水凝胶; 可视识别; 磷酸根; 钳型三联吡啶锌络合物; 选择性
吴彦 , 张扬 , 吴嘉杰 , 周俊 , 刘鸿 , 涂涛 . 通过金属有机小分子水凝胶选择性形成实现磷酸根的可视识别[J]. 有机化学, 2018 , 38(3) : 705 -709 . DOI: 10.6023/cjoc201709048
Due to high selective coordination ability of phosphate anion to the Zn center of pincer type terpyridine zinc nitrate complexes, intriguing visual recognition of phosphate anion via selective metallo-hydrogel formation has been successfully realized. Based on this interesting result, a series of control experiments were conducted including the study on thermodynamic stability, rheological property and microscopic morphology of the metallo-hydrogel, a plausible mechanism of the discrimination was proposed.

[1] Cui, K. Y.; Wang, Y.; Zhang, X. Jilin Normal Univ. J. (Nat. Sci. Ed.) 2004, 25, 50(in Chinese). (崔克宇, 王悦, 张祥, 吉林师范大学学报(自然科学版), 2004, 25, 50.)
[2] Hu, W. X.; Lin, L. R.; Huang, R. B.; Zheng, L. S. Chin. J. Inorg. Chem. 2009, 25, 381(in Chinese). (胡望霞, 林丽榕, 黄荣彬, 郑兰荪, 无机化学学报, 2009, 25, 381.)
[3] Miller, L. V.; Hambidge, K. M.; Fennessey, P. V. Anal. Chim. Acta 1990, 241, 249.
[4] Han, M. S.; Kim, D. H. Angew. Chem., Int. Ed. 2002, 41, 3809.
[5] Lee, K. H.; Lee, H. Y.; Lee, D. H.; Hong, J. I. Tetrahedron Lett. 2001, 42, 5447.
[6] Beer, P. D.; Gale, P. A. Angew. Chem., Int. Ed. 2001, 40, 486.
[7] Song, Y.; Li, Y.; Liu, Y. L.; Su, X. G.; Ma, Q. Talanta 2015, 144, 680.
[8] Dai, C.; Yang, C.; Yan, X. Anal. Chem. 2015, 87, 11455.
[9] Li, L.; Shang, G. L.; Qin, W. Analyst 2016, 141, 4573.
[10] Qin, J.; Li, D. X.; Miao, Y. M.; Yan, G. Q. RSC Adv. 2017, 7, 46657.
[11] Yu, G.; Yan, X.; Han, C.; Huang, F. Chem. Soc. Rev. 2013, 42, 6697.
[12] Steed, J. W. Chem. Soc. Rev. 2010, 39, 3686.
[13] Tu, T.; Fang, W. W.; Sun, Z. M. Adv. Mater. 2013, 25, 5304.
[14] Piepenbrock, M. O. M.; Clarke, N.; Steed, J. W. Langmuir 2009, 25, 8451.
[15] Shen, J. S.; Li, D. H.; Cai, Q. G.; Jiang, Y. B. J. Mater. Chem. 2009, 19, 6219.
[16] Bhowmik, S.; Ghosh, B. N.; Marjomáki, V.; Rissanen, K. J. Am. Chem. Soc. 2014, 136, 5543.
[17] Yamaguchi, S.; Yoshimura, I.; Kohira, T.; Tamaru, S.; Hamachi, I. J. Am. Chem. Soc. 2005, 127, 11835.
[18] Fang, W. W.; Liu, C.; Chen, J. B.; Lu, Z. W.; Li, Z. M.; Bao, X. L.; Tu, T. Chem. Commun. 2015, 51, 4267.
[19] Fang, W. W.; Liu, C.; Lu, Z. W.; Sun, Z. M.; Tu, T. Chem. Commun. 2014, 50, 10118.
[20] Fang, W. W.; Liu, X. Y.; Lu, Z. W.; Tu, T. Chem. Commun. 2014, 50, 3313.
[21] Fang, W. W.; Sun, Z. M.; Tu, T. J. Phys. Chem. C 2013, 117, 25185.
[22] Tu, T.; Fang, W. W.; Bao, X. L.; Li, X. B.; Karl, H. D. Angew. Chem., Int. Ed. 2011, 50, 6601.
/
| 〈 |
|
〉 |