

增强型超分子荧光探针对拉帕替尼的识别及其在细胞成像中的应用
收稿日期: 2017-11-09
修回日期: 2018-01-19
网络出版日期: 2018-03-08
基金资助
国家自然科学基金(No.21562015)、贵州省优秀青年科技人才培养计划(No.[2017]5636)、贵州省科学技术基金(No.[2014]2005)、贵州省普通高等学校科技拔尖人才支持计划(No.[2016]059)、贵州省科技厅联合基金(No.[2014]7658)、贵州大学研究生创新基金(No.2017001)资助项目.
A Turn-On Supramolecular Fluorescent Probe for Sensing Lapatinib and Its Application in Living Cell Imaging
Received date: 2017-11-09
Revised date: 2018-01-19
Online published: 2018-03-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21562015), the Excellent Youth Scientific Talents Foundation of Guizhou Province (No.[2017]5636), the Natural Science Fund of the Science and Technology Department of Guizhou Province (No. JZ-2014-2005), the Talents Support Project of Guizhou Province of Education (No. QJH-KY-2016-059), the Joint Funds Plan from the Science and Technology Department of Guizhou Province (No. QKH-LH-2014-7658), and the Graduate Student Innovative Funding of Guizhou University (No. 2017001).
王成会 , 唐青 , 席芸芸 , 杨梅 , 李涛 , 黄英 , 陶朱 . 增强型超分子荧光探针对拉帕替尼的识别及其在细胞成像中的应用[J]. 有机化学, 2018 , 38(6) : 1394 -1400 . DOI: 10.6023/cjoc201711018
aEngineering and Research Center for Southwest Bio-pharmaceutical Resources of National Education Ministry of China, Guizhou University, Guiyang 550025) (bKey Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang 550025) (cCollege of Tobacco Science, Guizhou University, Guiyang 550025
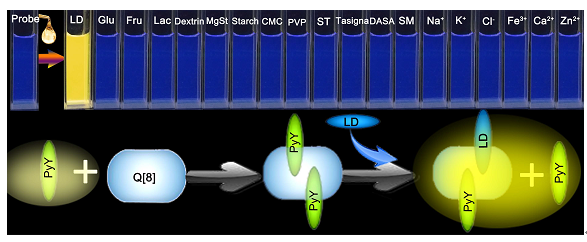
Key words: cucurbit[8]uril; pyronine Y; lapatinib ditosylate; fluorescent probe; cell imaging
[1] U S FDA. FDA approves Tykerb for advanced breast cancer patients[EB/OL].[2007-06-25]. http://www.fda.gov/bbs/topics/NEWS/2007/NEW01586.html.
[2] Devriese, L. A.; Koch, K. M.; Mergui-Roelvink, M.; Matthys, G. M.; Ma, W. W.; Robidoux, A.; Stephenson, J. J.; Chu, Q. S. C.; Oford, K. W.; Cartee, L.; Bothyl, J.; Arya, N.; Schellens, J. H. M. Invest. New Drugs 2014,32,481.
[3] Zhao, S.-J.; Zhu, Z. Chin. Pharm. J. 2008, 43, 1037(in Chinese) (赵胜军, 朱珠, 中国药学杂志, 2008, 43, 1037.)
[4] Feng, H.-R.; Li, Q.; Wang, H. L. Prog. Mod. Biomed. 2012, 12, 746(in Chinese). (冯焕荣, 李青, 王海琳, 现代生物医学进展, 2012, 12, 746.)
[5] Ohgami, M.; Homma, M.; Suzuki, Y.; Naito, K.; Yamada, M.; Mitsuhashi, S.; Fujisawa, F.; Kojima, H.; Kaburagi, T.; Uchiumi, K.; Yamada, Y.; Bando, H.; Hara, H.; Takei, K. Ther. Drug Monit. 2016, 6, 657.
[6] Roche, S.; McMahon, G.; Clynes, M.; O'Connor, R. J. Chromatogr. B 2009, 877, 3982.
[7] Lankheet, N. A.; Hillebrand, M. J.; Rosing, H.; Schellens, J. H.; Beijnen, J. H.; Huitema, A. D. Biomed. Chromatogr. 2013, 27, 466.
[8] Escudero-Ortiz, V.; Perez-Ruixo, J. J.; Valenzuela, B. Ther. Drug Monit. 2013, 6, 796.
[9] Liu, Y.; Ji, D. K.; Dong, L.; Galanos, N.; Zang, Y.; Li, J.; Vidal, S.; He, X. P. Chem. Commun. 2017, 53, 11937.
[10] Xu, M.; Wu, S.; Zeng, F.; Yu, C. Langmuir 2010, 26, 4529.
[11] Chen, Y.; Han, K. Y.; Liu, Y. Bioorg. Med. Chem. 2007, 15, 4537.
[12] Wang, K.; Guo, D. S.; Wang, X.; Liu, Y. ACS Nano 2011, 5, 2880.
[13] Valeur, B.; Leray, I. Inorg. Chim. Acta 2007, 360, 765.
[14] Ji, H. F.; Dabestani, R.; Brown, G. M. J. Am. Chem. Soc. 2000, 122, 9306.
[15] Leray, I.; Valeur, B. Eur. J. Inorg. Chem. 2009, 3525.
[16] Nau, W. M.; Ghale, G.; Hennig, A.; Bakirci, H.; Bailey, D. M. J. Am. Chem. Soc. 2009, 131, 11558.
[17] Li, F. S.; Xu, Y. Q.; Li, H. J.; Wang, C. X.; Lu, A. P.; Sun, S. G. New J. Chem. 2014, 38, 1396.
[18] Florea, M.; Nau, W. M. Angew. Chem., Int. Ed. 2011, 50, 9338.
[19] Dsouza, R. N.; Pischel, U.; Nau, W. M. Chem. Rev. 2011, 111, 7941.
[20] Hennig, A.; Bakirci, H.; Nau, W. M. Nat. Methods 2007, 4, 629.
[21] Sindelar, V.; Cejas, M. A.; Raymo, F. M.; Chen, W.; Parker, S. E.; Kaifer, A. E. Chem.-Eur. J. 2005, 11, 7054.
[22] Montes-Navajas, P.; Corma, A.; Garcia, H. ChemPhysChem 2008, 9, 713.
[23] Xi, Y.-Y.; Tang, Q.; Huang, Y.; Zhu, T.; Xue, S. F.; Zhu, Q. J. J. Guizhou Univ. (Nat. Sci.) 2016, 33, 35(in Chinese). (席芸芸, 唐青, 黄英, 陶朱, 薛赛凤, 祝黔江, 贵州大学学报(自然科学版), 2016, 33, 35.)
[24] Kim, H. J.; Heo, J.; Jeon, W. S.; Lee, E.; Kim, J.; Sakamoto, S.; Yamaguchi, K.; Kim, K. Angew. Chem., Int. Ed. 2001, 40, 1526.
[25] Baumes, L. A.; Sogo, M. B.; Navajas, P. M.; Corma, A.; Garcia, H. Tetrahedron Lett. 2009, 50, 7001.
[26] Baumes, L. A.; Buaki, M.; Jolly, J.; Corma, A.; Garcia, H. Tetrahedron Lett. 2011, 52, 1418.
[27] Moon, K.; Grindstaff, J.; Sobransingh, D.; Kaifer, A. E. Angew. Chem., Int. Ed. 2004, 43, 5496.
[28] Wang, W.; Kaifer, A. E. Angew. Chem., Int. Ed. 2006, 45, 7042.
[29] Day, A. I.; Blanch, R. J.; Arnold, A. P.; Lorenzo, S.; Lewis, G. R.; Dance, I. Angew. Chem., Int. Ed. 2002, 41, 275.
[30] Kubin, R. F.; Fletcher, A. N. J Lumin. 1982, 27, 455.
[31] Kubota, Y.; Steiner, R. F. Biophys. Chem. 1977, 6, 279.
/
| 〈 |
|
〉 |