

碳正离子介导的二氢喹啉的氧化碳氢炔基化
Trityl Ion-Mediated Oxidative C—H Alkynylation of 1, 2-Dihydroquinolines
Received date: 2018-03-01
Revised date: 2018-03-16
Online published: 2018-04-13
刘子强 , 赵冉 , 贺妮 , 李伟 . 碳正离子介导的二氢喹啉的氧化碳氢炔基化[J]. 有机化学, 2018 , 38(5) : 1261 -1266 . DOI: 10.6023/cjoc201803001
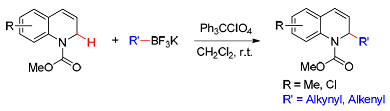
An efficient synthesis of α-substituted 1, 2-dihydroquinoline compounds through the oxidative C—H functionalization of N-acyl-dihydroquinoline with diverse organoboron reagents mediated by triphenylcarbium perchlorate (Ph3CClO4) is reported. The reaction exhibits good functional group tolerance, allowing for C—H alkynylation and alkenylation proceeding smoothly in good yields.
[1] (a) Michael, J. P. Nat. Prod. Rep. 2007, 24, 223.
(b) Michael, J. P. Nat. Prod. Rep. 2008, 25, 166.
(c) Welsch, M. E.; Snyder, S. A.; Stockwell, B. R. Curr. Opin. Chem. Biol. 2010, 14, 347.
[2] (a) Kouznetsov, V.; Palma, A.; Ewert, C.; Varlamov, A. J. Heterocycl. Chem. 1998, 35, 761.
(b) Sridharan, V.; Suryavanshi, P. A.; Menéndez, J. C. Chem. Rev. 2011, 111, 7157.
(c) Katritzky, A. R.; Rachwal, S.; Rachwal, B. Tetrahedron 1996, 52, 15031.
[3] (a) Takamura, M.; Funabashi, K.; Kanai, M.; M. Shibasaki, J. Am. Chem. Soc. 2001, 123, 6801.
(b) Yamaoka, Y.; Miyabe, H.; Takemoto, Y. J. Am. Chem. Soc. 2007, 129, 6686.
[4] (a) Graham, T. J. A.; Shields, J. D.; Doyle, A. G. Chem. Sci. 2011, 2, 980.
(b) Kodama, T.; Moquist, P. N.; Schaus, S. E. Org. Lett. 2011, 13, 6316.
(c) Sun, S.; Mao, Y.; Lou, H.; Liu, L. Chem. Commun. 2015, 51, 10691.
(d) Volla, C. M. R.; Fava, E.; Atodiresei, L.; Rueping, M. Chem. Commun. 2015, 51, 15788.
(e) Berti, F.; Malossi, F.; Marchettib, F.; Pineschi, M. Chem. Commun. 2015, 51, 13694.
[5] (a) Trost, B. M. Acc. Chem. Res. 2002, 35, 695.
(b) Wender, P. A.; Verma, V. A.; Paxton, T. J.; Pillow, T. H. Acc. Chem. Res. 2008, 41, 40.
[6] (a) Murahashi, S.; Zhang, D. Chem. Soc. Rev. 2008, 37, 1490.
(b) Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780.
(c) Li, C. J. Acc. Chem. Res. 2009, 42, 335.
(d) Sun, C. L.; Li, B. J.; Shi, Z. J. Chem. Rev. 2011, 111, 1293.
(e) Zhang, C.; Tang, C.; Jiao, N. Chem. Soc. Rev. 2012, 41, 3464.
(f) Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068.
(g) Liu, Z.; Chen, L.; Li, J.; Liu, K.; Zhao, J.; Xu, M.; Feng, L.; Wan, R.; Li, W.; Liu, L. Org. Biomol. Chem, 2017, 15, 7600.
(h) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215.
(i) Liu, X.; Sun, S.; Meng, Z.; Lou, H.; Liu, L. Org. Lett. 2015, 17, 2396.
(j) Xie, Z.; Zan, X.; Sun, S.; Pan, X.; Liu, L. Org. Lett. 2016, 18, 3944.
(k)Wang, G., Mao, Y., Liu, L. Org. Lett. 2016, 18, 6476.
(l) Zhang, Q.; Lv, J.; Luo, S. Acta Chim. Sinica 2016, 74, 61(in Chinese). (张启超, 吕健, 罗三中, 化学学报, 2016, 74, 61.)
(m) Qin, Y.; Zhu, L.; Luo, S. Chem. Rev. 2017, 117, 9433.
[7] (a) Murahashi, S.-I.; Komiya, N.; Terai, H.; Nakae, T. J. Am. Chem. Soc. 2003, 125, 15312.
(b) Murahashi, S.-I.; Nakae, T.; Terai, H.; Komiya, N. J. Am. Chem. Soc. 2008, 130, 11005.
(c) Boess, E.; Sureshkumar, D.; Sud, A.; Wirtz, C.; Farès, C.; Klussmann, M. J. Am. Chem. Soc. 2011, 133, 8106.
(d) Boess, E.; Schmitz, C.; Klussmann, M. J. Am. Chem. Soc. 2012, 134, 5317.
(e) Li, Z.; Li, C. J. J. Am. Chem. Soc. 2004, 126, 11810.
(f) Li, Z.; Yu, R.; Li, H. Angew. Chem., Int. Ed. 2008, 47, 7497.
(g) Yang, F.; Li, J.; Xie, J.; Huang, Z. Z. Org. Lett. 2010, 12, 5214.
(h) Muramatsu, W.; Nakano, K.; Li, C. J. Org. Lett. 2013, 15, 3650.
(i) Li, Z.; Li, C.-J. Org. Lett. 2004, 6, 4997.
(j) Zhang, G.; Zhang, Y.; Wang, R. Angew. Chem., Int. Ed. 2011, 50, 10429.
[8] (a) Guo, C.; Song, J.; Luo, S.-W.; Gong, L.-Z. Angew. Chem., Int. Ed. 2010, 49, 5558.
(b) Li, Z.; MacLeod, P. D.; Li, C.-J. Tetrahedron:Asymmetry 2006, 17, 590.
(c) Yang, Q.; Zhang, L.; Ye, C.; Luo, S.; Wu, L.-Z. Tung, C.-H. Angew. Chem., Int. Ed. 2017, 56, 3694.
(d) Neel, A. J.; Hehn, J. P.; Tripet, P. F.; Toste, F. D. J. Am. Chem. Soc. 2013, 135, 14044.
(e) Zhang, G.; Ma, Y.; Wang, S.; Zhang, Y.; Wang, R. J. Am. Chem. Soc. 2012, 134, 12334.
(f) Zhang, G.; Ma, Y.; Wang, S.; Kong, W.; Wang, R. Chem. Sci. 2013, 4, 2645.
(g) Bergonzini, G.; Schindler, C. S.; Wallentin, C.-J.; Jacobsen, E. N.; Stephenson, C. R. J. Chem. Sci. 2014, 5, 112.
[9] (a) Pintér, Á.; Sud, A.; Sureshkumar, D.; Klussmann, M. Angew. Chem., Int. Ed. 2010, 49, 5004.
(b) Liu, X.; Meng, Z.; Li, C.; Lou, H.; Liu, L. Angew. Chem., Int. Ed. 2015, 54, 6012.
(c) Richter, H.; Fröhlich, R.; Daniliuc, C.-G.; García Mancheño, O. Angew. Chem., Int. Ed. 2012, 51, 8656.
(d) Richter, H.; García Mancheño, O. Eur. J. Org. Chem. 2010, 4460.
(e) Ghobrial, M.; Harhammer, K.; Mihovilovic, M. D.; Schnürch, M. Chem. Commun. 2010, 46, 8836.
(f) Ghobrial, M.; Schnürch, M.; Mihovilovic, M. D. J. Org. Chem. 2011, 76, 8781.
(g) Liu, X.; Sun, B.; Xie, Z.; Qin, X.; Liu, L.; Lou, H. J. Org. Chem. 2013, 78, 3104.
(h) Sun, S.; Li, C.; Floreancig, P. E.; Lou, H.; Liu, L. Org. Lett. 2015, 17, 1684.
(i) Long, H.; Wang, G.; Lu, R.; Xu, M.; Zhang, K.; Qi, S.; He, Y.; Bu, Y.; Liu, L. Org. Lett. 2017, 19, 2146.
[10] (a) Xie, Z.; Liu, L.; Chen, W.; Zheng, H.; Xu, Q.; Yuan, H.; Lou, H. Angew. Chem., Int. Ed. 2014, 53, 3904.
(b) Chen, W.; Xie, Z.; Zheng, H.; Lou, H.; Liu, L. Org. Lett. 2014, 16, 5988.
(c) Wan, M.; Meng, Z.; Lou, H.; Liu, L. Angew. Chem., Int. Ed. 2014, 53, 13845.
[11] (a) Kim, H.; MacMillan, D. W. C. J. Am. Chem. Soc. 2008, 130, 398.
(b) Vo, C.-V. T.; Mitchell, T. A.; Bode, J. W. J. Am. Chem. Soc. 2011, 133, 14082.
(c) Fujiwara, Y.; Domingo, V.; Seiple, I. B.; Gianatassio, R.; Del Bel, M.; Baran, P. S. J. Am. Chem. Soc. 2011, 133, 3292.
(d) Nielsen, D. K.; Doyle, A. G. Angew. Chem., Int. Ed. 2011, 50, 6056.
/
| 〈 |
|
〉 |