

四溴化碳促进下2-(1H-苯并[d]咪唑)-3-芳基丙烯腈的高效合成
收稿日期: 2018-04-18
修回日期: 2018-06-13
网络出版日期: 2018-06-29
基金资助
国家自然科学基金(No.51403073)、江苏省高校面上(No.16KJB150006)及江苏省低维材料化学重点实验室开放基金(No.JSKC15145)资助项目.
CBr4-Promoted Efficient Synthesis of 2-(1H-Benzo[d] imidazol-2-yl)-3-arylacrylonitriles
Received date: 2018-04-18
Revised date: 2018-06-13
Online published: 2018-06-29
Supported by
Project supported by the National Natural Science Foundation of China (No. 51403073), the Department of Education of Jiangsu Province (No. 16KJB150006) and the Jiangsu Key Laboratory for Chemistry of Low-Dimensional Materials (No. JSKC15145).
2-苯并咪唑-3-芳基丙烯腈衍生物具有广谱的生物活性,同时还是一类重要的合成中间体,在有机合成领域具有广泛的应用.以芳醛和2-氰甲基苯并咪唑为原料,乙醇为溶剂,在四溴化碳促进下,高效合成了2-(1H-苯并[d]咪唑)-3-芳基丙烯腈.反应在回流条件下搅拌5~10 min即可完成,以74%~96%的产率得到目标产物.该方法为2-苯并咪唑-3-芳基丙烯腈衍生物的制备提供了反应条件温和、后处理方便和底物适用范围广的合成策略.
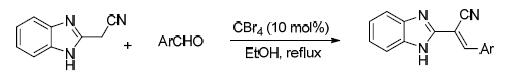
关键词: 苯并咪唑; 四溴化碳; 丙烯腈; Knoevenagel缩合
王翔 , 陈平 , 支三军 , 胡华友 , 阚玉和 . 四溴化碳促进下2-(1H-苯并[d]咪唑)-3-芳基丙烯腈的高效合成[J]. 有机化学, 2018 , 38(11) : 3123 -3126 . DOI: 10.6023/cjoc201804037
2-(1H-Benzo[d] imidazol-2-yl)-3-arylacrylonitrile derivatives not only exhibit a variety of important biological activities, but also are important intermediates in organic synthesis. The CBr4-promoted reaction of aromatic aldehydes with 2-(1H-benzo[d] imidazol-2-yl) acetonitrile to obtain 2-(1H-benzo[d] imidazol-2-yl)-3-arylacrylonitriles was developed. Structurally diverse 2-(1H-benzo[d] imidazol-2-yl)-3-arylacrylonitriles were obtained in moderate to good yields (74%~96%) under mild conditions. This method has the advantages of operational simplicity and wide substrate scope.
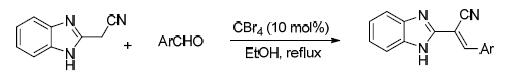
Key words: benzimidazol; carbon tetrabromide; acrylonitrile; Knoevenagel condensation
[1] (a) Yu, N.; Aramini, J. M.; Germann, M. W.; Huang, Z. Tetrahedron Lett. 2000, 41, 6993.
(b) Hou, H.; Li, Z.; Ying, A.; Xu, S. Chin. J. Org. Chem. 2014, 34, 1277(in Chinese). (侯海亮, 李志峰, 应安国, 许松林, 有机化学, 2014, 34, 1277.)
[2] Tietze, L. F.; Rackelmann, N. Pure Appl. Chem. 2004, 76, 1967.
[3] (a) Kozaki, M.; Isoyama, A.; Kogen, A. A.; Okada, K. Org. Lett. 2005, 7, 115.
(b) Krebs, F. C.; Spanggaard, H. J. Org. Chem. 2002, 67, 7185.
[4] Li, Q.; Xiang, S.; Mao, S.; Ren, Y. Chin. J. Org. Chem. 2017, 37, 608(in Chinese). (李强根, 向仕凯, 毛双, 任译, 有机化学, 2017, 37, 608.)
[5] Chen, Z.; Liu, X.-K.; Zheng, C.-J.; Ye, J.; Liu, C.-L.; Li, F.; Ou, X.-M.; Lee, C.-S.; Zhang, X.-H. Chem. Mater. 2015, 27, 5206.
[6] Oksana, T.; Nicolas, M.; Chiara, B. J. Mater. Chem. C 2016, 4, 5940.
[7] Tmur, G.; Andre, F.; Teulon J.-M.; Daniel, P.; Michele, C.; Alix, C. J. Med. Chem. 1992, 35, 4455.
[8] Hasegawa, M.; Nishigaki, N.; Washio, Y.; Kano, K.; Harris, P. A.; Sato, H.; Mori, I.; West, R. I.; Shibahara, M.; Toyoda, H.; Wang, L.; Nolte, R. T.; Veal, J. M.; Cheung, M. J. Med. Chem. 2007, 50, 4453.
[9] Refaat, H. M. Eur. J. Med. Chem. 2010, 45, 2949.
[10] Hranjec, M.; Pavlovic, G.; Marjanovic, M.; Kralj, M.; Karminski-Zamola, G. Eur. J. Med. Chem. 2010, 45, 2405.
[11] Mugnaini, C.; Rajamaki, S.; Tintori, C.; Corelli, F.; Massa, S.; Witvrouw, M.; Debyser, Z.; Veljkovic, V.; Botta, M. Bioorg. Med. Chem. Lett. 2007, 17, 5370.
[12] Hsu, W.-S.; Tsai, M.-H.; Barve, I. J.; Yellol, G. S.; Sun, C.-M. ACS Comb. Sci. 2017, 19, 492.
[13] Panda, K.; Suresh, J. R.; Ila, H.; Junjappa, H. J. Org. Chem. 2003, 68, 3498.
[14] Narhe, B. D.; Tsai, M.-H.; Sun, C.-M. ACS Comb. Sci. 2014, 16, 421.
[15] (a) Gao, X.; Gao, C.; Gao, R. Asian J. Chem. 2015, 27, 2145.
(b) Srinivas, K.; Katari, N. K.; Karuna, P. S.; Surendra Babu, M. S. Curr. Organocatal. 2015, 2, 44.
(c) Hranjec, M.; Karminski-Zamola, G. Molecules 2007, 12, 1817.
[16] Dubey, P. K.; Reddy, P. V. V. P. Indian J. Heterocycl. Chem. 2007, 16, 395.
[17] Kazi, I.; Guha, S.; Sekar, G. Org. Lett. 2017, 19, 1244.
[18] Kishore Babu, P. N.; Rama Devi, B.; Dubey, P. K. Chem. Sin. 2013, 4, 107.
[19] Saczewski, F.; Reszka, P; Gdaniec, M; Gruenert, R; Bednarski, P. J. J. Med. Chem. 2004, 47, 3438.
/
| 〈 |
|
〉 |