

海洋天然核苷Kipukasin D的首次全合成
收稿日期: 2018-06-19
修回日期: 2018-07-11
网络出版日期: 2018-08-23
基金资助
国基自然科学基金(Nos.21462019,21676131)、江西省科技厅重点项目(No.20143ACB20012)和江西省教育厅科技(No.170673)资助项目.
First Total Synthesis of Marine Natural Nucleoside Kipukasin D
Received date: 2018-06-19
Revised date: 2018-07-11
Online published: 2018-08-23
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21462019, 21676131), the Bureau of Science & Technology of Jiangxi Province (No. 20143ACB20012) and the Education Department of Jiangxi Province (No. 170673).
丁海新 , 李闯 , 董祥有 , 曹伴鹏 , 张宁 , 洪三国 , 肖强 . 海洋天然核苷Kipukasin D的首次全合成[J]. 有机化学, 2018 , 38(12) : 3351 -3355 . DOI: 10.6023/cjoc201806028
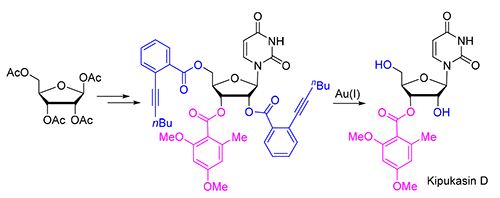
The first total synthesis of marine natural nucleoside kipukasin D was disclosed in 9 steps and 15.7% overall yield using commercially available tetra-O-acetyl-β-D-ribose as starting material. In Vorbrüggen glycosylation, ortho-iodinebenzoate acted as neighboring participating group leading to β-nucleoside. The key step was selectively deprotection of 2'-O and 5'-O ortho-alkynylbenzoates by freshing prepared Ph3PAuOTFA in the presence of ethanol (6 equiv.) and H2O (1 equiv.) in dichloromethane (DCM). The reaction condition of our newly developed approach is very mild and neutral, which effectively avoids the transesterification between 2'-OH and 3'-OH. The result further demonstrat that ortho-alkynylbenzoate is an orthogonal protecting group compatible with other ester groups, which could find wide applications in nucleoside and carbohydrate chemistry.
Key words: nucleoside; neighboring-group; transesterification; catalysis; total synthesis
[1] (a) Haefner, B. Drug Discovery Today 2003, 8, 536.
(b) Molinski, T. F.; Dalisay, D. S.; Lievens, S. L.; Saludes, J. P. Nat. Rev. Drug Discovery 2009, 8, 69.
[2] (a) Shi, Q.-W.; Li, L.-G.; Wang, Y.-F. Drug Eval. Res. 2010, 33, 165(in Chinese). (史清文, 李力更, 王于方, 药物评价研究, 2010, 33, 165.)
(b) Wang, S.; Luan, H.; Wu, C.-M.; Guo, P. J. Int. Pharm. Res. 2014, 41, 537(in Chinese). (王帅, 栾虹, 吴崇明, 郭鹏, 国际药学研究杂志, 2014, 41, 537.)
[3] Sun, K.; Li, Y.; Guo, L.; Wang, Y.; Liu, P.; Zhu, W. Mar. Drugs 2014, 12, 3970.
[4] Sugimoto, K.; Sadahiro, Y.; Kagiyama, I.; Kato, H.; Scherman, D. H.; Williams, R. M.; Tsukamoto, S. Tetrahedron Lett. 2017, 58, 2797.
[5] Wang, H.; Lu, Z.; Qu, H.; Liu, P.; Miao, C.; Zhu, T.; Li, J.; Hong, K.; Zhu, W. Arch. Pharmacal Res. 2012, 35, 1387.
[6] Yang, G.; Sanjo, L.; Yun, K.; Leutou, A. S.; Kim, G. D.; Choi, H. D.; Kang, J. S.; Hong, J.; Son, B. W. Chem. Pharm. Bull. 2011, 59, 1174.
[7] (a) Facey, P. C.; Porter, R. B.; Laatsch, H. Planta Med. 2016, 81, 1.
(b) Liu, S.; Dai, H.; Konuklugil, B.; Orfali, R. S.; Lin, W.; Kalscheuer, R.; Proksch, P. Phytochem. Lett. 2016, 18, 187.
[8] Jiao, P.; Mudur, S. V.; Gloer, J. B.; Wicklow, D. T. J. Nat. Prod. 2007, 70, 1308.
[9] Chen, M.; Fu, X. M.; Kong, C. J.; Wang, C. Y. Nat. Prod. Res. 2014, 28, 895.
[10] Zhuravleva, O. I.; Kirichuk, N. N.; Denisenko, V. A.; Dimtrenok, P. S.; Pivkin, M. V.; Afiyatullov, S. S. Chem. Nat. Compd. 2016, 52, 266.
[11] (a) Cao, B.; Ding, H.; Yang, R.; Wang, X.; Xiao, Q. Mar. Drugs 2012, 10, 1412.
(b) Sun, J.; Dou, Y.; Ding, H.; Yang, R.; Sun, Q.; Xiao, Q. Mar. Drugs 2012, 10, 881.
(c) Hu, C.; Ruan, Z.; Ding, H.; Zhou, Y.; Xiao, Q. Molecules 2017, 22, 643.
[12] (a) Huang, H.-Y.; Ruan, Z.-Z.; Hu, T.; Xiao, Q. Chin. J. Org. Chem. 2014, 34, 1358(in Chinese). (黄海洋, 阮志忠, 胡韬, 肖强, 有机化学, 2014, 34, 1358.)
(b) Sun, Z.-D.; Zhu, Y.-L.; Huang, H.-Y.; Song, X.-R.; Xiao, Q. Chin. J. Org. Chem. 2016, 36, 2729(in Chinese). (孙志东, 朱云龙, 黄海洋, 宋贤荣, 肖强, 有机化学, 2016, 36, 2729.)
[13] Li, C.; Ding, H.; Ruan, Z.; Zhou, Y.; Xiao, Q. Beilstein J. Org. Chem. 2017, 13, 855.
[14] Jackson, M. D.; Denu, J. M. J. Biol. Chem. 2002, 277, 18535.
[15] Dvorakova, M.; Pribylova, M.; Pohl, R.; Migaud, M. E.; Vanek, T. Tetrahedron 2012, 68, 6701.
[16] Hasegawa, H.; Akira, K.; Shinohara, Y.; Kasuya, Y.; Hashimoto, T. Biol. Pharm. Bull. 2001, 24, 852.
[17] Ding, H.; Li, C.; Zhou, Y.; Hong, S.; Zhang, N.; Xiao, Q. RSC Adv. 2017, 7, 1814.
/
| 〈 |
|
〉 |