

PhI(OAc)2参与的β-酮酸酯及β-酮酰胺亲核氟化反应
收稿日期: 2018-08-31
修回日期: 2018-10-25
网络出版日期: 2018-12-05
基金资助
国家自然科学基金(No.21402067)、江苏省自然科学基金(No.BK20170569)、江苏省高校自然科学基金(No.17KJB150013)资助项目.
Fluorination of β-Ketoesters and β-Ketoamides Based on PhI(OAc)2
Received date: 2018-08-31
Revised date: 2018-10-25
Online published: 2018-12-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 21402067), the Natural Science Foundation of Jiangsu Province (No. BK20170569) and the Natural Science Fund for Colleges and Universities in Jiangsu Province (No. 17KJB150013).
吴文胜 , 袁航 , 黄高魁 , 蒋春辉 , 陆鸿飞 . PhI(OAc)2参与的β-酮酸酯及β-酮酰胺亲核氟化反应[J]. 有机化学, 2019 , 39(1) : 137 -143 . DOI: 10.6023/cjoc201808047
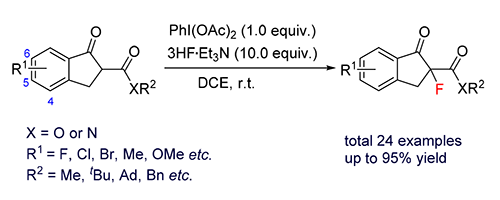
Herein, a nucleophilic fluorination reaction to construct fluorine-containing β-ketoesters and β-ketoamides is reported. The reaction uses PhI(OAc)2 as oxidant and 3HF·Et3N as fluorinating reagent. It can effectively build a series of fluorochemical compounds containing quaternary carbon center under room temperature reaction conditions for 30 min. Compared with the traditional electrophilic fluorination reaction, this method has the advantages of no metal participation, short reaction time, simple reaction conditions and high reaction yield.
[1] (a) Isanbor, C.; Hagan, D. O. J. Fluorine Chem. 2006, 127, 303.
(b) Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013.
(c) Purser, S.; Moore, P. R.; Swallow, S.; Gouvemeur, V. Chem. Soc. Rev. 2008, 37, 320.
(d) Jiang, W.; Sanchez-Rosello, M.; Acena, J. L.; Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Hong, L. Chem. Rev. 2014, 114, 2432.
(e) Xu, J. X.; Hu, Y. L.; Huang, D. F.; Wang, K. H.; Xu, C. M.; Niu, T. Adv. Synth. Catal. 2012, 354, 515.
(f) Koike, T.; Akita, M. J. Fluorine Chem. 2014, 167, 30.
(g) Wang, X.; Liu, G. K.; Xu, X. H.; Naoyuki, S.; Etsuko, T.; Norio, S. Angew. Chem., Int. Ed. 2014, 53, 1827.
(h) Sugiishi, K.; Matsugi, M.; Hamamoto, H.; Amii, H. RSC. Adv. 2015, 5, 17269.
(i) Köckinger, M.; Ciaglia, T.; Bersier, M.; Hanselmann, P.; Gutmann, B.; Kappe, C. O. Green Chem. 2018, 20, 108.
[2] (a) Zhao, Y. M.; Cheung, M. S.; Lin, Z. Y.; Sun, J. W. Angew. Chem., Int. Ed. 2012, 51, 10359.
(b) Suzuki, S.; Kitamura, Y.; Lectard, S.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2012, 51, 4581.
(c) Shibatomi, K.; Soga, Y.; Narayama, A.; Fujisawa, I.; Iwasa, S. J. Am. Chem. Soc. 2012, 134, 9836.
(d) Ma, B. W.; Lin, X. B.; Lin, L. L.; Feng, X. M.; Liu, X. H. J. Org. Chem. 2017, 82, 701.
(e) Salomon, P.; Zard, S. Z. Org. Lett. 2014, 16, 1482.
(f) Qi, X. X.; Yu, F.; Chen, P. H.; Liu, G. S. Angew. Chem., Int. Ed. 2017, 56, 12692.
(g) Molnár, I. G.; Gilmour, R. J. Am. Chem. Soc. 2016, 138, 5004.
(h) Ma, X. H.; Diane, M.; Ralph, G.; Chen, C.; Biscoe, M. R. Angew. Chem., Int. Ed. 2017, 56, 12663.
(i) Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem., Int. Ed. 2013, 52, 8214.
[3] (a) Shibatomi, K.; Tsuzuki, Y.; Iwasa, S. Chem. Lett. 2008, 37, 1098.
(b) Shibatomi, K.; Kitahara, K.; Sasaki, N.; Kawasaki, Y.; Fujisawa, I.; Iwasa, S. Nat. Commun. 2017, 8, 15600.
(c) Prusov, E. V. Angew. Chem., Int. Ed. 2017, 56, 14356.
(d) Kang, S. H.; Kim, D. Y. Adv. Synth. Catal. 2010, 352, 2783.
(e) Kitamura, T.; Kuriki, S.; Morshed, M. H.; Hori, Y. Org. Lett. 2011, 13, 2392.
(f) Kitamura, T.; Muta, K.; Muta, K. J. Org. Chem. 2014, 79, 5842.
[4] (a) Li, C. K.; Breit, B. J. Am. Chem. Soc. 2014, 136, 862.
(b) Turnbull, B. W. H.; Evans, P. A. J. Am. Chem. Soc. 2015, 137, 6156.
(c) Roy, S. R.; Didier, D.; Kleiner, A.; Marek, I. Chem. Sci. 2016, 7, 5989.
(d) Starkov, P.; Moore, J. T.; Duquette, D. C.; Stoltz, B. M.; Marek, I. J. Am. Chem. Soc. 2017, 139, 9615.
(e) Liu, J. Y.; Zhao, J.; Zhang, J. L.; Xu, P. F. Org. Lett. 2017, 19, 1846.
[5] (a) Gu, X.; Zhang, Y.; Xu, Z. J.; Che, C. M. Chem. Commun. 2014, 50, 7870.
(b) Wang, Y. F.; Wang, H. J.; Jiang, Y. D.; Zhang, C.; Shao, J. J.; Xu, D. Q. Green. Chem. 2017, 19, 1674.
(c) Zheng, L. S.; Wei, Y. L.; Jiang, K. Z.; Deng, Y.; Zheng, Z. J.; Xu, L. W. Adv. Synth. Catal. 2014, 356, 3769.
(d) Kwon, S. J.; Kim, D. Y. J. Fluorine Chem. 2015, 180, 201.
[6] Hintermann, L.; Togni, A. T. Angew. Chem., Int. Ed. 2000, 39, 4359.
[7] Wang, X. S.; Lan, Q.; Shirakawa, S.; Maruoka, K. Chem. Commun. 2010, 46, 321.
[8] (a) Nash, T. J.; Pattison, G. Eur. J. Org. Chem. 2015, 3779.
(b) Suzuki, S.; Kamo, T.; Fukushi, K.; Hiramatsu, T.; Tokunaga, E.; Dohi, T.; Kita, Y.; Shibata, N. Chem. Sci. 2014, 5, 2754.
(c) Pluta, R.; Krach, P. E.; Cavallo, L.; Falvienne, L.; Rueping, M. ACS Catal. 2018, 8, 2582.
(d) Wang, Y.; Yuan, H.; Lu, H. F.; Zheng, W. H. Org. Lett. 2018, 20, 2555.
(e) Goldbberg, N. W.; Shen, X.; Li, J. K.; Ritter, T. Org. Lett. 2016, 18, 6102.
(f) Woerly, E. M.; Banik, S. M.; Jacobsen, E. N. J. Am. Chem. Soc. 2016, 138, 13858.
(g) Satoshi, M.; Hiroki, O.; Ayako, K.; Kanami, K.; Yuki, M.; Jun, I.; Kodai, N.; Ryo, H.; Toshiya, U.; Kenya, C. Org. Lett. 2017, 19, 2572.
[9] Gondo, K; Kitamura, T. Molecules 2012, 17, 6625.
[10] (a) Kitamura, T.; Muta, K.; Muta, K. J. Org. Chem. 2014, 79, 5842.
(b) Wirth, T. Angew. Chem., Int. Ed. 2005, 44, 3656.
(c)Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328.
(d) Zhang, B. B.; Zhang, X.; Hu, B.; Sun, D. S.; Wang, S. L.; Zhang-Negrerie, D.; Du, Y. F. Org. Lett. 2017, 19, 902.
[11] Uyanik, M.; Yasui, T.; Ishihara, K. J. Org. Chem. 2017, 82, 11946.
[12] Banik, S. M.; Medley, J. W.; Jacobsen, E. N. J. Am. Chem. Soc. 2018, 138, 5000.
[13] Kitamura, T.; Muta, K.; Oyamada, J. J. Org. Chem. 2015, 80, 10431.
/
| 〈 |
|
〉 |