

手性磷酰胺配体不对称催化二芳基甲醇类化合物的合成
Asymmetric Synthesis of Diarylmethanols by Chiral Phosphoramide Ligands Catalysts
Received date: 2019-02-24
Revised date: 2019-03-29
Online published: 2019-05-21
为了获得具有重要生理活性的手性二芳基甲醇类化合物, 以R,R-1,2-环己二胺为原料获得了一系列磷酰胺配体, 并系统考察了该类配体在芳基烷基锌对芳香醛的不对称加成反应中的催化活性, 在优化的反应条件下, 即在30 mol%磷酰胺配体N-((1R,2R)-2-(异丙基氨基)环己基)-P,P-二苯基磷酰胺(9c)存在下, 可以高达94%的ee值(对映体过量值)及大于90%收率获得相应的手性二芳基甲醇类化合物. 尽管催化剂用量较大, 在该体系中, 配体可非常方便回收再利用. 同时, 对反应机理进行了推测, 认为反应过程所形成的四元过渡态和六元过渡态, 有利于提高反应的对映选择性.
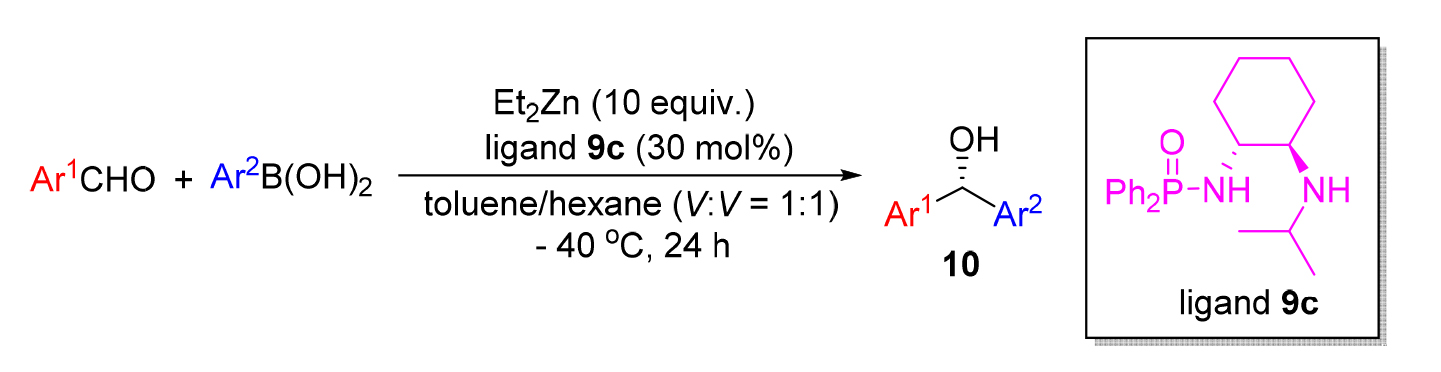
关键词: 手性磷酰胺类配体; 手性二芳基甲醇类化合物; 不对称催化
郭庆君 . 手性磷酰胺配体不对称催化二芳基甲醇类化合物的合成[J]. 有机化学, 2019 , 39(10) : 2912 -2919 . DOI: 10.6023/cjoc201902026
In order to improve the application of chiral phosphoramide ligands in catalytic asymmetric reactions, thiophosphoramide, which was synthesized from trans-1,2-cyclohexanediamine was used as a catalyst to synthesize chiral diarylmethanol compounds through addition reaction. The catalytic activity of the ligand in the asymmetric addition reaction of the arylalkyl zincs to the aromatic aldehyde can be as high as 94% ee under the optimized reaction conditions in the presence of 30 mol% phosphoramide ligand N-((1R,2R)-2-(isopropylamino)cyclohexyl)-P,P-diphenylphosphinic amide (9c) and the corresponding chiral diarylmethanol compound was obtained with the yields of >90%. Despite the large amount of catalyst, the ligand is very convenient to recycle and reuse in this system. At the same time, the reaction mechanism was speculated, and it is believed that the quaternary transition state and the six-element transition state formed by the reaction process are beneficial to improve the enantioselectivity of the reaction.
| [1] | Einhorn, A. Ber. Dtsch. Chem. Ges. 1883, 16, 2208. |
| [2] | Torrens, A.; Castrillo, J.; Claparols, A.; Redondo, J . Synlett 1999, 765. |
| [3] | (a) Casy, A. F.; Drake, A. F.; Ganellin, C. R.; Mercer, A. D.; Upton, C . Chirality 1992, 4, 356. |
| [3] | (b) Müller, P.; Nury, P.; Bernardinelli, G . Eur. J. Org. Chem. 2001, 2001, 4137. |
| [4] | Hite, G.; Barouh, V.; Dall, H.; Patel, D. J. Med. Chem. 1971, 14, 834. |
| [5] | Guo, Z.; Raeissi, S.; White, R. B.; Stevens, J. C. Drug Metab. Dispos. 1997, 25, 390. |
| [6] | Li, C.; Chauret, N.; Trimble, L. A.; Nicoll-Griffith, D. A; Silva, J. M; MacDonald, D.; Perrier, H.; Yergey, J. A.; Parton, T.; Alexander, R. P.; Warrellow, G. J. Drug Metab. Dispos. 2001, 29, 232. |
| [7] | (a) James, M. N. G.; Williams, G. J. B. Can. J. Chem. 1974, 52 1872. |
| [7] | (b) Shafi'ee, A.; Hite, G. J. Med. Chem. 1969, 12, 266. |
| [8] | (a) Bolm, C.; Hildebrand, J. P.; Mu?iz, K.; Hermanns, N. Angew. Chem., Int. Ed. 2001, 40 3284. |
| [8] | (b) Ji, J. X.; Wu, J.; Xu, L. J.; Chiu, W. Y.; Kim, H. L.; Albert, S. C. Pure Appl. Chem. 2006; 78, 267. |
| [9] | (a) Fernández-Mateos, E.; Maciá, B.; Yus, M. Tetrahedron: Asymmetry 2012, 23, 789. |
| [9] | (b) Wu, K. H.; Gau, H. M. J. Am. Chem. Soc. 2006, 128 14808. |
| [10] | (a) Da, C.-S.; Wang, J. R.; Yin, X. G.; Fan, X. Y.; Liu, Y.; Yu, S. L . Org. Lett. 2009, 11 5578. |
| [10] | (b) Fan, X. Y.; Yang, Y. X.; Zhuo, F.-F.; Yu, S. L.; Li, X.; Guo, Q. P.; Du, Z. X.; Da, C. S . Chem.-Eur. J. 2010, 16 7988. |
| [11] | Tomita, D.; Wada, R.; Kanai, M.; Shibasaki, M . J. Am. Chem. Soc. 2005, 127, 4138. |
| [12] | Chang, S. J.; Zhou, S.; Gau, H. M . RSC Adv. 2015, 5, 9368. |
| [13] | (a) Fernández-Mateos, E.; Maciá, B.; Yus, M . Eur. J. Org. Chem. 2012, 3732. |
| [13] | (b) Nakagawa, Y.; Muramatsu, Y.; Harada, T . Eur. J. Org. Chem. 2010, 6535. |
| [14] | Bolm, C.; Rudolph, J . J. Am. Chem. Soc. 2002, 124, 14850. |
| [15] | Bauer, M.; Maurer, F.; Hoffmann, S. M.; Kazmaier, U . Synlett 2008, 3203. |
| [16] | (a) Ding, M.; Zhou, F.; Liu, Y. L.; Wang, C. H.; Zhao, X. L.; Zhou, J. Chem. Sci. 2011, 2, 2035. |
| [16] | (b) Dong, J.; Du, D. M. Org. Biomol. Chem. 2012, 10, 8125. |
| [16] | (c) Yu, Y. N.; Xu, M. H . Acta Chim. Sinica 2017, 75, 655 (in Chinese). |
| [16] | ( 于月娜, 徐明华 , 化学学报, 2017, 75, 655.) |
| [17] | Huang, H.; Bian, G.; Zong, H.; Wang, Y.; Yang, S.; Yue, H.; Song, L.; Fan, H . Org. Lett. 2016, 18, 2524. |
| [18] | Shen, B.; Huang, H.; Bian, G.; Zong, H.; Song, L. Chirality 2013, 25, 561. |
| [19] | Huang, H. Y.; Zong, H.; Shen, B.; Yue, H. F.; Bian, G. L.; Song, L. Tetrahedron 2014, 70, 1289. |
| [20] | Kaik, M.; Gawroński, J . Tetrahedron: Asymmetry 2003 14 1559. |
| [21] | Huang, H. Y.; Z ong, H.; Bian, G. L.; Song, L. J. Org. Chem. 2012, 77, 10427. |
| [22] | Yang, Y. X.; Liu, Y.; Zhang, L.; Jia, Y. E.; Wang, P.; Zhuo, F. F.; An, X. T.; Da, C. S. J. Org. Chem. 2014, 79, 10696. |
| [23] | Wu, X. Y.; Liu, X. Y.; Zhao, G. Tetrahedron: Asymmetry 2005, 16, 2299. |
| [24] | Tian, C.; Gong, L.; Meggers, E. . Chem. Commun 2016, 52, 4207. |
| [25] | Barsamian, A. L.; Wu, Z. H.; Blakemore, P. R. Org. Biomol. Chem. 2015, 13, 3781. |
| [26] | Wang, Y. B.; Zong, H.; Huang, H. Y.; Song, L . Tetrahedron: Asymmetry. 2017, 28 90. |
/
| 〈 |
|
〉 |