有机化学 ›› 2019, Vol. 39 ›› Issue (10): 2912-2919.DOI: 10.6023/cjoc201902026 上一篇 下一篇
研究论文
收稿日期:2019-02-24
修回日期:2019-03-29
发布日期:2019-05-21
通讯作者:
郭庆君
E-mail:guoqingjun2005@126.com
Received:2019-02-24
Revised:2019-03-29
Published:2019-05-21
Contact:
Guo, Qingjun
E-mail:guoqingjun2005@126.com
文章分享
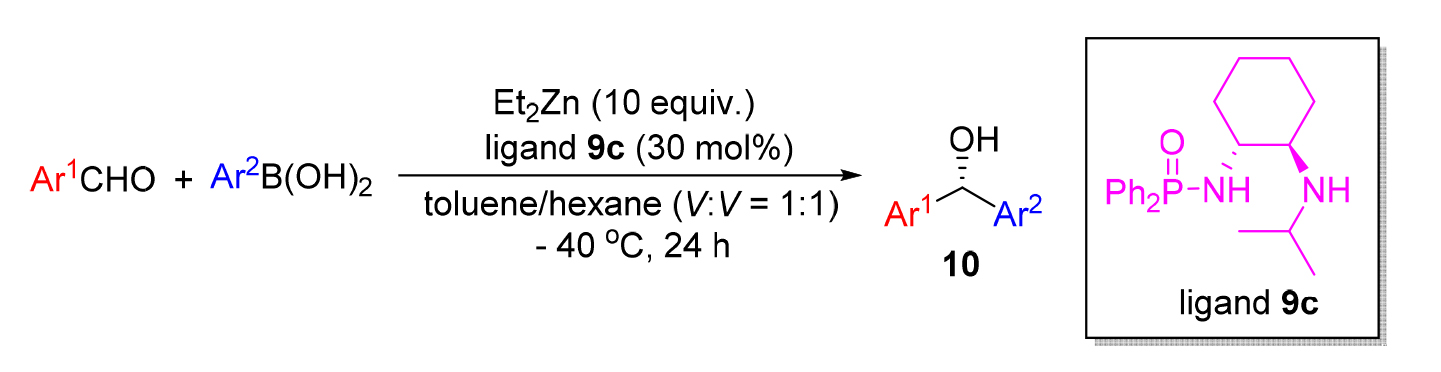
为了获得具有重要生理活性的手性二芳基甲醇类化合物, 以R,R-1,2-环己二胺为原料获得了一系列磷酰胺配体, 并系统考察了该类配体在芳基烷基锌对芳香醛的不对称加成反应中的催化活性, 在优化的反应条件下, 即在30 mol%磷酰胺配体N-((1R,2R)-2-(异丙基氨基)环己基)-P,P-二苯基磷酰胺(9c)存在下, 可以高达94%的ee值(对映体过量值)及大于90%收率获得相应的手性二芳基甲醇类化合物. 尽管催化剂用量较大, 在该体系中, 配体可非常方便回收再利用. 同时, 对反应机理进行了推测, 认为反应过程所形成的四元过渡态和六元过渡态, 有利于提高反应的对映选择性.
郭庆君. 手性磷酰胺配体不对称催化二芳基甲醇类化合物的合成[J]. 有机化学, 2019, 39(10): 2912-2919.
Guo, Qingjun. Asymmetric Synthesis of Diarylmethanols by Chiral Phosphoramide Ligands Catalysts[J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2912-2919.
| Entry | Ligand | X | NR1R2 | Yieldb/% | eec/% |
|---|---|---|---|---|---|
| 1 | 7 | O | — | 83 | 46 |
| 2 | 8 | O | NHiPr | 89 | 68 |
| 3 | 9a | O | NHBn | 96 | 29 |
| 4 | 9b | O | NEt2 | 84 | 56 |
| 5 | 9c | O | NHiPr | 93 | 78 |
| 6 | 9d | S | NHiPr | 88 | 76 |
| Entry | Ligand | X | NR1R2 | Yieldb/% | eec/% |
|---|---|---|---|---|---|
| 1 | 7 | O | — | 83 | 46 |
| 2 | 8 | O | NHiPr | 89 | 68 |
| 3 | 9a | O | NHBn | 96 | 29 |
| 4 | 9b | O | NEt2 | 84 | 56 |
| 5 | 9c | O | NHiPr | 93 | 78 |
| 6 | 9d | S | NHiPr | 88 | 76 |
| Entry | 9c/ mol% | R | Temp./ ℃ | Solvent | Yieldb/% | eec/% |
|---|---|---|---|---|---|---|
| 1 | 20 | Me | 0~25 | Toluene | 89 | 74 |
| 2 | 20 | Me | 0~25 | Hexane | 85 | 70 |
| 3d | 20 | Me | 0~25 | Hexane/MTBE | 53 | 71 |
| 4d | 20 | Me | 0~25 | Hexane/THF | Trace | N.D.e |
| 5d | 20 | Me | 0~25 | Toluene/hexane | 93 | 78 |
| 6d | 20 | Me | –10 | Toluene/hexane | 90 | 80 |
| 7d | 20 | Me | –20 | Toluene/hexane | 90 | 82 |
| 8d | 20 | Me | –40 | Toluene/hexane | 89 | 86 |
| 9d | 20 | Et | –40 | Toluene/hexane | 90 | 89 |
| 10d, f | 30 | Et | –40 | Toluene/hexane | 93 | 94 |
| 11d | 40 | Et | –40 | Toluene/hexane | 93 | 92 |
| Entry | 9c/ mol% | R | Temp./ ℃ | Solvent | Yieldb/% | eec/% |
|---|---|---|---|---|---|---|
| 1 | 20 | Me | 0~25 | Toluene | 89 | 74 |
| 2 | 20 | Me | 0~25 | Hexane | 85 | 70 |
| 3d | 20 | Me | 0~25 | Hexane/MTBE | 53 | 71 |
| 4d | 20 | Me | 0~25 | Hexane/THF | Trace | N.D.e |
| 5d | 20 | Me | 0~25 | Toluene/hexane | 93 | 78 |
| 6d | 20 | Me | –10 | Toluene/hexane | 90 | 80 |
| 7d | 20 | Me | –20 | Toluene/hexane | 90 | 82 |
| 8d | 20 | Me | –40 | Toluene/hexane | 89 | 86 |
| 9d | 20 | Et | –40 | Toluene/hexane | 90 | 89 |
| 10d, f | 30 | Et | –40 | Toluene/hexane | 93 | 94 |
| 11d | 40 | Et | –40 | Toluene/hexane | 93 | 92 |
| Entry | Product | 10 | Yieldb/% | eec/% | Entry | Product | 10 | Yieldb/% | eec/% | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |  | (R)-10a | 93 | 94 | 12 |  | (R)-10l | 84 | 86 | |
| 2 |  | (R)-10b | 98 | 84 | 13 |  | (S)-10i | 93 | 88 | |
| 3 |  | (R)-10c | 92 | 92 | 14 |  | (S)-10c | 90 | 89 | |
| 4 |  | (R)-10d | 88 | 90 | 15 |  | (S)-10l | 95 | 92 | |
| 5 |  | (R)-10e | 83 | 87 | 16 |  | (R)-10m | 92 | 92 | |
| 6 |  | (R)-10f | 81 | 93 | 17 |  | (R)-10n | 82 | 87 | |
| 7 |  | (R)-10g | 86 | 82 | 18 |  | (S)-10o | 95 | 90 | |
| 8 |  | (R)-10h | 91 | 90 | 19 |  | (S)-10p | 96 | 84 | |
| 9 |  | (R)-10i | 92 | 89 | 20 |  | (R)-10q | 91 | 93 | |
| 10 |  | (R)-10j | 90 | 91 | 21 |  | (R)-10r | 95 | 84 | |
| 11 |  | (R)-10k | 85 | 91 | 22 |  | (R)-10s | 90 | 53 |
| Entry | Product | 10 | Yieldb/% | eec/% | Entry | Product | 10 | Yieldb/% | eec/% | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |  | (R)-10a | 93 | 94 | 12 |  | (R)-10l | 84 | 86 | |
| 2 |  | (R)-10b | 98 | 84 | 13 |  | (S)-10i | 93 | 88 | |
| 3 |  | (R)-10c | 92 | 92 | 14 |  | (S)-10c | 90 | 89 | |
| 4 |  | (R)-10d | 88 | 90 | 15 |  | (S)-10l | 95 | 92 | |
| 5 |  | (R)-10e | 83 | 87 | 16 |  | (R)-10m | 92 | 92 | |
| 6 |  | (R)-10f | 81 | 93 | 17 |  | (R)-10n | 82 | 87 | |
| 7 |  | (R)-10g | 86 | 82 | 18 |  | (S)-10o | 95 | 90 | |
| 8 |  | (R)-10h | 91 | 90 | 19 |  | (S)-10p | 96 | 84 | |
| 9 |  | (R)-10i | 92 | 89 | 20 |  | (R)-10q | 91 | 93 | |
| 10 |  | (R)-10j | 90 | 91 | 21 |  | (R)-10r | 95 | 84 | |
| 11 |  | (R)-10k | 85 | 91 | 22 |  | (R)-10s | 90 | 53 |
| [1] |
Einhorn, A. Ber. Dtsch. Chem. Ges. 1883, 16, 2208.
doi: 10.1002/(ISSN)1099-0682 |
| [2] |
Torrens, A.; Castrillo, J.; Claparols, A.; Redondo, J . Synlett 1999, 765.
doi: 10.1055/s-1999-2712 |
| [3] |
(a) Casy, A. F.; Drake, A. F.; Ganellin, C. R.; Mercer, A. D.; Upton, C . Chirality 1992, 4, 356.
doi: 10.1002/(ISSN)1520-636X |
|
(b) Müller, P.; Nury, P.; Bernardinelli, G . Eur. J. Org. Chem. 2001, 2001, 4137.
doi: 10.1002/(ISSN)1520-636X |
|
| [4] |
Hite, G.; Barouh, V.; Dall, H.; Patel, D. J. Med. Chem. 1971, 14, 834.
doi: 10.1021/jm00291a014 |
| [5] | Guo, Z.; Raeissi, S.; White, R. B.; Stevens, J. C. Drug Metab. Dispos. 1997, 25, 390. |
| [6] | Li, C.; Chauret, N.; Trimble, L. A.; Nicoll-Griffith, D. A; Silva, J. M; MacDonald, D.; Perrier, H.; Yergey, J. A.; Parton, T.; Alexander, R. P.; Warrellow, G. J. Drug Metab. Dispos. 2001, 29, 232. |
| [7] |
(a) James, M. N. G.; Williams, G. J. B. Can. J. Chem. 1974, 52 1872.
doi: 10.1139/v74-267 |
|
(b) Shafi'ee, A.; Hite, G. J. Med. Chem. 1969, 12, 266.
doi: 10.1139/v74-267 |
|
| [8] |
(a) Bolm, C.; Hildebrand, J. P.; Muñiz, K.; Hermanns, N. Angew. Chem., Int. Ed. 2001, 40 3284.
doi: 10.1002/(ISSN)1521-3773 |
|
(b) Ji, J. X.; Wu, J.; Xu, L. J.; Chiu, W. Y.; Kim, H. L.; Albert, S. C. Pure Appl. Chem. 2006; 78, 267.
doi: 10.1002/(ISSN)1521-3773 |
|
| [9] | (a) Fernández-Mateos, E.; Maciá, B.; Yus, M. Tetrahedron: Asymmetry 2012, 23, 789. |
| (b) Wu, K. H.; Gau, H. M. J. Am. Chem. Soc. 2006, 128 14808. | |
| [10] |
(a) Da, C.-S.; Wang, J. R.; Yin, X. G.; Fan, X. Y.; Liu, Y.; Yu, S. L . Org. Lett. 2009, 11 5578.
doi: 10.1021/ol9020942 |
|
(b) Fan, X. Y.; Yang, Y. X.; Zhuo, F.-F.; Yu, S. L.; Li, X.; Guo, Q. P.; Du, Z. X.; Da, C. S . Chem.-Eur. J. 2010, 16 7988.
doi: 10.1021/ol9020942 |
|
| [11] |
Tomita, D.; Wada, R.; Kanai, M.; Shibasaki, M . J. Am. Chem. Soc. 2005, 127, 4138.
doi: 10.1021/ja0507362 |
| [12] |
Chang, S. J.; Zhou, S.; Gau, H. M . RSC Adv. 2015, 5, 9368.
doi: 10.1039/C4RA14173C |
| [13] | (a) Fernández-Mateos, E.; Maciá, B.; Yus, M . Eur. J. Org. Chem. 2012, 3732. |
| (b) Nakagawa, Y.; Muramatsu, Y.; Harada, T . Eur. J. Org. Chem. 2010, 6535. | |
| [14] |
Bolm, C.; Rudolph, J . J. Am. Chem. Soc. 2002, 124, 14850.
doi: 10.1021/ja028518l |
| [15] | Bauer, M.; Maurer, F.; Hoffmann, S. M.; Kazmaier, U . Synlett 2008, 3203. |
| [16] | (a) Ding, M.; Zhou, F.; Liu, Y. L.; Wang, C. H.; Zhao, X. L.; Zhou, J. Chem. Sci. 2011, 2, 2035. |
| (b) Dong, J.; Du, D. M. Org. Biomol. Chem. 2012, 10, 8125. | |
| (c) Yu, Y. N.; Xu, M. H . Acta Chim. Sinica 2017, 75, 655 (in Chinese). | |
| ( 于月娜, 徐明华 , 化学学报, 2017, 75, 655.) | |
| [17] |
Huang, H.; Bian, G.; Zong, H.; Wang, Y.; Yang, S.; Yue, H.; Song, L.; Fan, H . Org. Lett. 2016, 18, 2524.
doi: 10.1021/acs.orglett.6b00088 |
| [18] |
Shen, B.; Huang, H.; Bian, G.; Zong, H.; Song, L. Chirality 2013, 25, 561.
doi: 10.1002/chir.22171 |
| [19] |
Huang, H. Y.; Zong, H.; Shen, B.; Yue, H. F.; Bian, G. L.; Song, L. Tetrahedron 2014, 70, 1289.
doi: 10.1016/j.tet.2013.12.054 |
| [20] | Kaik, M.; Gawroński, J . Tetrahedron: Asymmetry 2003 14 1559. |
| [21] |
Huang, H. Y.; Z ong, H.; Bian, G. L.; Song, L. J. Org. Chem. 2012, 77, 10427.
doi: 10.1021/jo3016715 |
| [22] |
Yang, Y. X.; Liu, Y.; Zhang, L.; Jia, Y. E.; Wang, P.; Zhuo, F. F.; An, X. T.; Da, C. S. J. Org. Chem. 2014, 79, 10696.
doi: 10.1021/jo502070r |
| [23] | Wu, X. Y.; Liu, X. Y.; Zhao, G. Tetrahedron: Asymmetry 2005, 16, 2299. |
| [24] |
Tian, C.; Gong, L.; Meggers, E. . Chem. Commun 2016, 52, 4207.
doi: 10.1039/C6CC00972G |
| [25] |
Barsamian, A. L.; Wu, Z. H.; Blakemore, P. R. Org. Biomol. Chem. 2015, 13, 3781.
doi: 10.1039/C5OB00159E |
| [26] | Wang, Y. B.; Zong, H.; Huang, H. Y.; Song, L . Tetrahedron: Asymmetry. 2017, 28 90. |
| [1] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [2] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [3] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [4] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [5] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [6] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [7] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| [8] | 方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083. |
| [9] | 赵佳怡, 葛怡聪, 何川. 不对称催化Si—H/X—H脱氢偶联构筑硅中心手性[J]. 有机化学, 2023, 43(10): 3352-3366. |
| [10] | 曾燕, 叶飞. 不对称催化构建硅立体中心化合物的新反应体系研究进展[J]. 有机化学, 2023, 43(10): 3388-3413. |
| [11] | 代增进, 张绪穆, 殷勤. 铵盐为胺源的不对称还原胺化反应研究进展[J]. 有机化学, 2022, 42(8): 2261-2274. |
| [12] | 李晖, 殷亮. 铜催化的直接型插烯反应研究进展[J]. 有机化学, 2022, 42(6): 1573-1585. |
| [13] | 吴逾诸, 申盼盼, 段文增, 马玉道. 卡宾催化对亚甲基苯醌的不对称硼化反应的研究[J]. 有机化学, 2022, 42(5): 1483-1492. |
| [14] | 徐萌萌, 蔡泉. 2-吡喃酮的催化不对称Diels-Alder反应研究进展[J]. 有机化学, 2022, 42(3): 698-713. |
| [15] | 陈运荣, 刘炜, 杨晓瑜. 叔醇的动力学拆分研究进展[J]. 有机化学, 2022, 42(3): 679-697. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
