

含硫醚三唑的1,4-戊二烯-3-酮衍生物合成及生物活性研究
收稿日期: 2019-03-23
修回日期: 2019-04-18
网络出版日期: 2019-06-12
基金资助
国家重点研发计划(2017YFD0200506);国家自然科学基金资助项目(21867003)
Syntheses and Biological Activities of 1,4-Pentadien-3-oneDerivatives Containing Thioether Triazole Moiety
Received date: 2019-03-23
Revised date: 2019-04-18
Online published: 2019-06-12
Supported by
Project supported by the National Key Research and Development Program of China(2017YFD0200506);The National Natural Science Foundation of China(21867003)
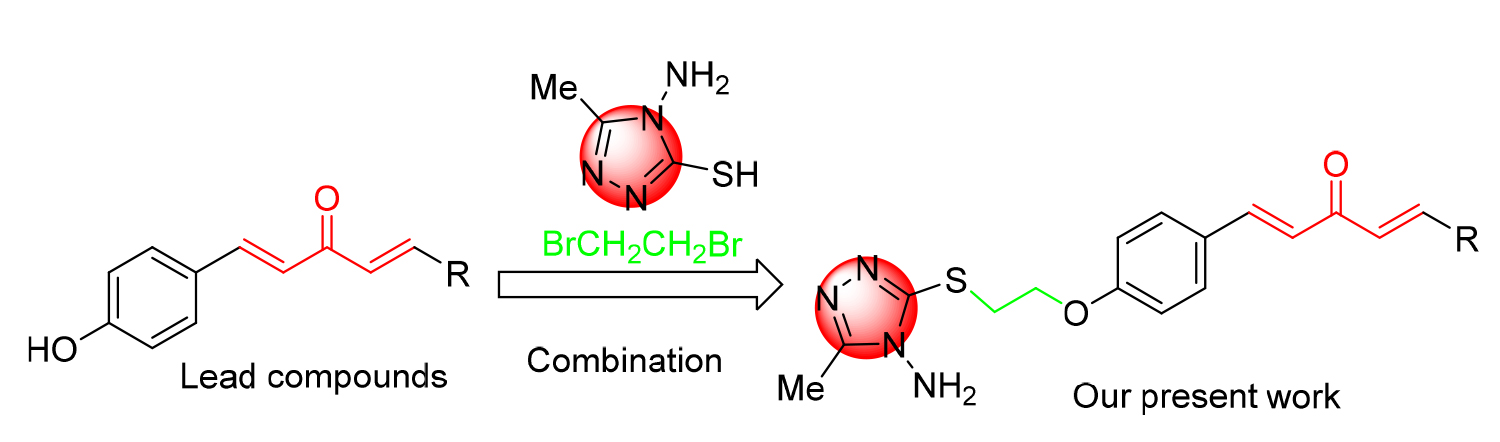
将含硫醇的三唑引入到1,4-戊二烯-3-酮结构中, 合成一系列含硫醚三唑的1,4-戊二烯-3-酮类衍生物, 其结构通过 1H NMR、 13C NMR、HRMS进行表征. 生物活性测试结果表明: 目标化合物对柑橘溃疡病菌(X. citri)、水稻白叶枯病菌(X. oryzae)、烟草青枯病菌(R. solanacearum)都表现出一定的抑制活性. 其中, 化合物F4、F6、F16对柑橘溃疡病菌的EC50值分别为16.3、9.9、15.9 μg/mL, 优于对照药叶枯唑(54.9 μg/mL); 化合物F1、F7、F15对水稻白叶枯病菌的EC50值分别为9.6、19.2、21.3 μg/mL, 优于对照药叶枯唑(69.3 μg/mL); 化合物F3、F6对烟草青枯病菌的EC50值分别为14.2、14.5 μg/mL, 优于对照药叶枯唑(82.6 μg/mL). 通过扫描电镜成像探讨了目标化合物F6对柑橘溃疡病菌(X. Citri)的可能抑菌机制.
关键词: 1,4-戊二烯-3-酮; 硫醚三唑; 生物活性; 扫描电镜
陈英 , 李普 , 陈梅 , 苏时军 , 贺军 , 张敏 , 柳立伟 , 薛伟 . 含硫醚三唑的1,4-戊二烯-3-酮衍生物合成及生物活性研究[J]. 有机化学, 2019 , 39(10) : 2813 -2820 . DOI: 10.6023/cjoc201903048
A series of novel 1,4-pentadien-3-one derivatives containing thioether triazole units were synthesized by introducing triazoles bearing thiol groups into the structures of 1,4-pentadien-3-one. The structures of the newly synthesized compounds were assigned via 1H NMR, 13C NMR and HRMS. Bioassays indicated that some of the compounds showed potential antibacterial activities against X. citri, X. oryzae and R. solanacearum. Among them, compounds F4, F6 and F16 demonstrated appreciable inhibitory effect against Xanthomonas axonopodis pv. citri, with half-maximal effective concentration (EC50) values of 16.3, 9.9 and 15.9 μg/mL, which were better than commercial agent bismerthiazol (54.9 μg/mL). Compounds F1, F7 and F15 demonstrated appreciable inhibitory effects against Xanthomonas oryzae pv. Oryzae with EC50 values of 9.6, 19.2 and 21.3 μg/mL, which were better than commercial agent bismerthiazol (69.3 μg/mL). Compounds F3 and F6 also demonstrated appreciable inhibitory effects against Ralstonia solanacearum with EC50 values of 14.2 and 14.5 μg/mL, which were better than commercial agent bismerthiazol (82.6 μg/mL). The possible mechanism of the antibacterial activity of the target compound F6 against Xanthomonas axonopodis was investigated through scanning electron microscopy.

| [1] | Chen, L.-J.; Li, P.; Wang, X.-B.; Ruan, X.-H.; Xue, W. Chem. Bull. 2017, 80, 1156(in Chinese). |
| [1] | ( 陈丽娟, 李普, 王晓斌, 阮祥辉, 薛伟, 化学通报, 2017, 80, 1156.) |
| [2] | Ren, Y. H.; Jin, H.; Tao, K.; Hou, T. P . Mol. Cell. Toxicol. 2015, 11, 187. |
| [3] | Peron, F.; Lazarin-Bidóia, D.; Din, Z. U.; Rodrigues-Filho, E.; Ueda-Nakamura, T.; Silva, S. O.; Nakamura.V, C. Biomed. Res. Int. 2017, 1. |
| [4] | Wu, J.; Zhu, Y. Y.; Zhao, Y. H.; Shan, W. L.; Hu, D. Y.; Chen, J.X. Chin. Chem. Lett. 2016, 27, 948. |
| [5] | Chen, C. L.; Chen, J.; Gu, H. Y.; Bao, N.; Dai, H. Molecules 2017, 22, 1126. |
| [6] | Zhou, J.; Tao, Q. Q.; Wang, P. Y.; Shao, W. B.; Wu, Z. B.; Li, Z. Bioorg. Med. Chem. Lett. 2018, 28, 1742. |
| [7] | Zhu, H. P.; Xu, T. T.; Qiu, C. Y.; Wu, B. B.; Zhang, Y. L.; Chen, L.F. Eur. J. Med. Chem. 2016, 121, 181. |
| [8] | Wang, Z. S.; Chen, L. Z.; Zhou, H. P.; Liu, X. H; Chen, F.H. . Bioorg. Med. Chem. Lett. 2017, 27, 1803. |
| [9] | Luo, H.; Yang, S. J.; Cai, Y. Q.; Peng, Z. J.; Liu, T. Eur. J. Med. Chem. 2014, 84, 746. |
| [10] | Badr, G.; Gul, H. I.; Yamali, C.; Mohamed, A. A. M.; Badr, B. M.; Gul, M. . Bioorg. Chem. 2018, 78, 46. |
| [11] | Luo, H.; Yang, S. J.; Hong, D.; Xue, W.; Xie, P. Chem. Cent. J. 2017, 11, 23. |
| [12] | Xue, W.; Gong, H.-Y.; Qiu, Q.-J.; Zhao, H.-J.; Li, H.-C.; Han, F.-F. Chem. Reag. 2013, 35, 201 (in Chinese). |
| [12] | ( 薛伟, 龚华玉, 仇秋娟, 赵洪菊, 李海畅, 韩菲菲, 化学试剂, 2013, 35, 201.) |
| [13] | Zhang, J.-P.; Li, P.; Wang, Y.-H.; Zhang, C.; Chen, L.-J.; Tang, X.; He, M.; Xue, W . Chem. J. Chin. Univ 2018, 39, 1455(in Chinese). |
| [13] | ( 张菊平, 李普, 王一会, 张橙, 陈丽娟, 汤旭, 贺鸣, 薛伟 , 高等学校化学学报, 2018, 39, 1455.) |
| [14] | Kulaba?, N.; Tatar, E.; ?zakp?nar, ?. B.; ?zsavc?, D.; Pannecouque, C.; Clercq, E. D Kü?ükgüzel, ?. .; Eur. J. Med. Chem. 2016, 121, 58. |
| [15] | Somagond, S. M.; Kamble, R. R.; Kattimani, P. P.; Shaikh, S. K. J.; Dixit, S. R.; Joshi, S.D. ChemistrySelect 2018, 3, 2004. |
| [16] | Wang, X. B.; Zhong, X. M.; Zhu, X. S.; Wang, H.; Li, Q.; Zhang, J. P.; Xue, W . Chem. Pap. 2017, 71, 1953. |
| [17] | Zhai, Z.-W.; Wang, Q.; Shen, Z.-H.; Tan, C.-X.; Weng, J.-Q Liu, X.-H. .; Chin. J. Org. Chem. 2017, 37, 232(in Chinese). |
| [17] | ( 翟志文, 汪乔, 沈钟华, 谭成侠, 翁建全, 刘幸海, 有机化学 , 2017, 37, 232.) |
| [18] | Ba?aran, E.; Karakü?ük-Iyido?an, A.; Schols, D.; Oru?-Emre, E. E. Chirality 2016, 28, 495. |
| [19] | Jin, R. Y.; Liu, J. L.; Zhang, G. H.; Li, J. J.; Zhang, S.; Guo, H . Chem. Biodiversity 2018. |
| [20] | Xu, F. Z.; Shao, J. H.; Zhu, Y. Y.; Liu, L. W.; Zhao, Y. H.; Shan, W. L . Chem. Pap. 2017, 71, 729. |
| [21] | Du, H.; Fan, Z.-J.; Yang, L.; Bao, X.-P. Chin. J. Org. Chem. 2018, 38, 531 (in Chinese). |
| [21] | ( 杜欢, 范治江, 杨岚, 鲍小平, 有机化学 , 2018, 38, 531.) |
| [22] | Yan, B.-R.; Lü, X.-Y.; Du, H.; Bao, X.-P. Chin. J. Org. Chem. 2016, 36, 207(in Chinese). |
| [22] | ( 闫柏任, 吕新阳, 杜欢, 鲍小平, 有机化学, 2016, 36, 207.) |
| [23] | Lin, G.-S.; Chen, Z.-C.; Duan, W.-G.; Wang, X.-Y.; Lei, F.-H . Chin. J. Org. Chem. 2018, 38, 2085(in Chinese). |
| [23] | ( 林桂汕, 陈智聪, 段文贵, 王晓宇, 雷福厚, 有机化学, 2018, 38, 2085.) |
| [24] | Zhou, J.M.S. . Thesis, Guizhou University,Guiyang , 2018 (in Chinese). |
| [24] | ( 周建, 硕士论文, 贵州大学 , 贵阳, 2018.) |
| [25] | Liu, C.-Y.; Zhao, Q.-Q.; Li, J . Chem. Reag. 2001, 23, 344(in Chinese). |
| [25] | ( 柳翠英, 赵全芹, 李娟, 化学试剂, 2001, 23, 344.) |
| [26] | Gan, X. H.; Hu, D. Y.; Li, P.; Wu, J.; Chen, X. W.; Xue, W.; Song, B.A. Pest Manage. Sci. 2015, 72, 534. |
| [27] | Wu, F.; Li, P.; Hu, D. Y.; Song, B.A. Res. Chem. Intermed. 2016, 42, 7153. |
| [28] | Xu, W. M.; Han, F. F.; He, M.; Hu, D. Y.; He, J.; Yang, S.; Song, B.A. J. Agric. Food Chem. 2012, 60, 1036. |
/
| 〈 |
|
〉 |