有机化学 ›› 2019, Vol. 39 ›› Issue (10): 2813-2820.DOI: 10.6023/cjoc201903048 上一篇 下一篇
研究论文
陈英, 李普, 陈梅, 苏时军, 贺军, 张敏, 柳立伟, 薛伟*( )
)
收稿日期:2019-03-23
修回日期:2019-04-18
发布日期:2019-06-12
通讯作者:
薛伟
E-mail:wxue@gzu.edu.cn
基金资助:
Chen Ying, Li Pu, Chen Mei, Su Shijun, He Jun, Zhang Min, Liu Liwei, Xue Wei*( )
)
Received:2019-03-23
Revised:2019-04-18
Published:2019-06-12
Contact:
Xue Wei
E-mail:wxue@gzu.edu.cn
Supported by:文章分享
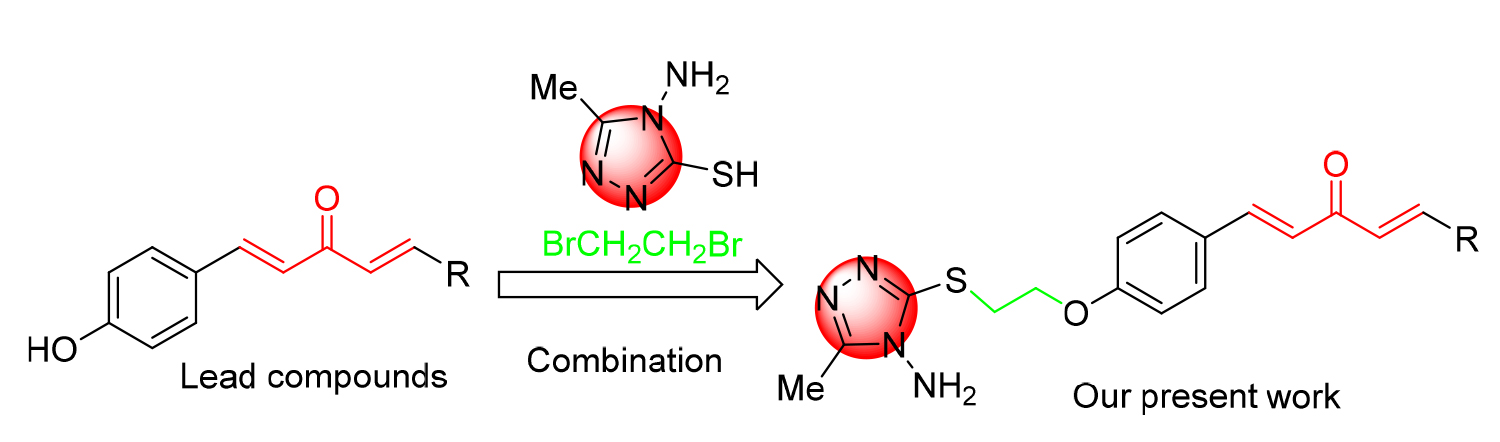
将含硫醇的三唑引入到1,4-戊二烯-3-酮结构中, 合成一系列含硫醚三唑的1,4-戊二烯-3-酮类衍生物, 其结构通过 1H NMR、 13C NMR、HRMS进行表征. 生物活性测试结果表明: 目标化合物对柑橘溃疡病菌(X. citri)、水稻白叶枯病菌(X. oryzae)、烟草青枯病菌(R. solanacearum)都表现出一定的抑制活性. 其中, 化合物F4、F6、F16对柑橘溃疡病菌的EC50值分别为16.3、9.9、15.9 μg/mL, 优于对照药叶枯唑(54.9 μg/mL); 化合物F1、F7、F15对水稻白叶枯病菌的EC50值分别为9.6、19.2、21.3 μg/mL, 优于对照药叶枯唑(69.3 μg/mL); 化合物F3、F6对烟草青枯病菌的EC50值分别为14.2、14.5 μg/mL, 优于对照药叶枯唑(82.6 μg/mL). 通过扫描电镜成像探讨了目标化合物F6对柑橘溃疡病菌(X. Citri)的可能抑菌机制.
陈英, 李普, 陈梅, 苏时军, 贺军, 张敏, 柳立伟, 薛伟. 含硫醚三唑的1,4-戊二烯-3-酮衍生物合成及生物活性研究[J]. 有机化学, 2019, 39(10): 2813-2820.
Chen Ying, Li Pu, Chen Mei, Su Shijun, He Jun, Zhang Min, Liu Liwei, Xue Wei. Syntheses and Biological Activities of 1,4-Pentadien-3-oneDerivatives Containing Thioether Triazole Moiety[J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2813-2820.
| Compound | R | X. citri | X. oryzae | R. solanacearum | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | |||||
| F1 | Ph | 47.0±1.6 | 36.5±4.0 | 89.5±0.9 | 74.3±0.1 | 54.5±3.4 | 49.8±5.3 | |||
| F2 | Thiophen-2-yl | 42.4±2.3 | 39.0±5.0 | 34.7±0.6 | 1.5±0.2 | 32.8±2.1 | 28.3±0.1 | |||
| F3 | 3,4-(CH3)2C6H3 | 57.4±4.1 | 41.9±2.7 | 17.7±0.8 | 10.6±1.4 | 75.9±1.5 | 62.4±0.4 | |||
| F4 | 4-NO2C6H4 | 72.3±5.3 | 62.8±2.8 | 58.0±0.5 | 41.6±1.2 | 51.6±0.1 | 47.9±0.5 | |||
| F5 | 4-ClC6H4 | 68.0±0.9 | 52.3±2.5 | 60.5±1.1 | 48.8±0.5 | 48.3±2.4 | 35.2±1.6 | |||
| F6 | 4-FC6H4 | 82.5±0.2 | 78.6±2.9 | 59.0±4.7 | 50.6±3.0 | 76.8±5.4 | 66.3±3.5 | |||
| F7 | 2,4-(CH3O)2C6H3 | 71.3±0.9 | 63.5±0.4 | 70.0±0.4 | 62.9±0.9 | 53.2±0.6 | 50.0±3.2 | |||
| F8 | 3-NO2C6H4 | 66.9±6.4 | 57.1±1.1 | 60.1±1.6 | 51.7±1.8 | 50.5±3.8 | 41.7±5.2 | |||
| F9 | 2-ClC6H4 | 72.6±4.3 | 64.1±2.2 | 56.2±4.7 | 47.6±2.6 | 41.6±0.9 | 35.2±0.5 | |||
| F10 | 4-CH3OC6H4 | 70.9±2.9 | 64.5±5.4 | 61.7±0.9 | 57.4±2.6 | 48.3±2.5 | 32.4±3.5 | |||
| F11 | 4-Methylthiazol-5-yl | 63.3±5.6 | 56.5±1.2 | 46.9±2.8 | 44.7±0.8 | 44.9±1.6 | 36.3±1.3 | |||
| F12 | Furan-2-yl | 55.3±1.3 | 48.1±2.5 | 68.6±4.2 | 56.7±0.2 | 37.4±0.7 | 23.2±4.8 | |||
| F13 | 3,4-(CH3O)2C6H3 | 38.5±4.0 | 25.0±1.6 | 66.2±0.4 | 60.4±3.9 | 54.8±1.9 | 42.1±1.3 | |||
| F14 | 4-BrC6H4 | 73.6±6.1 | 65.8±2.7 | 62.2±0.7 | 54.9±0.4 | 50.2±0.1 | 47.1±7.2 | |||
| F15 | 2,4-Cl2C6H3 | 56.7±2.4 | 45.7±4.9 | 61.7±0.4 | 54.9±0.6 | 55.9±1.1 | 42.4±0.6 | |||
| F16 | 4-CH3C6H4 | 77.4±4.0 | 68.7±1.4 | 53.0±1.9 | 44.6±0.6 | 51.0±1.8 | 33.9±2.3 | |||
| Bismerthiazolb | — | 59.5±7.7 | 49.5±5.7 | 56.0±1.2 | 33.8±2.0 | 61.1±6.2 | 49.5±5.3 | |||
| Compound | R | X. citri | X. oryzae | R. solanacearum | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | 50 μg/mL | |||||
| F1 | Ph | 47.0±1.6 | 36.5±4.0 | 89.5±0.9 | 74.3±0.1 | 54.5±3.4 | 49.8±5.3 | |||
| F2 | Thiophen-2-yl | 42.4±2.3 | 39.0±5.0 | 34.7±0.6 | 1.5±0.2 | 32.8±2.1 | 28.3±0.1 | |||
| F3 | 3,4-(CH3)2C6H3 | 57.4±4.1 | 41.9±2.7 | 17.7±0.8 | 10.6±1.4 | 75.9±1.5 | 62.4±0.4 | |||
| F4 | 4-NO2C6H4 | 72.3±5.3 | 62.8±2.8 | 58.0±0.5 | 41.6±1.2 | 51.6±0.1 | 47.9±0.5 | |||
| F5 | 4-ClC6H4 | 68.0±0.9 | 52.3±2.5 | 60.5±1.1 | 48.8±0.5 | 48.3±2.4 | 35.2±1.6 | |||
| F6 | 4-FC6H4 | 82.5±0.2 | 78.6±2.9 | 59.0±4.7 | 50.6±3.0 | 76.8±5.4 | 66.3±3.5 | |||
| F7 | 2,4-(CH3O)2C6H3 | 71.3±0.9 | 63.5±0.4 | 70.0±0.4 | 62.9±0.9 | 53.2±0.6 | 50.0±3.2 | |||
| F8 | 3-NO2C6H4 | 66.9±6.4 | 57.1±1.1 | 60.1±1.6 | 51.7±1.8 | 50.5±3.8 | 41.7±5.2 | |||
| F9 | 2-ClC6H4 | 72.6±4.3 | 64.1±2.2 | 56.2±4.7 | 47.6±2.6 | 41.6±0.9 | 35.2±0.5 | |||
| F10 | 4-CH3OC6H4 | 70.9±2.9 | 64.5±5.4 | 61.7±0.9 | 57.4±2.6 | 48.3±2.5 | 32.4±3.5 | |||
| F11 | 4-Methylthiazol-5-yl | 63.3±5.6 | 56.5±1.2 | 46.9±2.8 | 44.7±0.8 | 44.9±1.6 | 36.3±1.3 | |||
| F12 | Furan-2-yl | 55.3±1.3 | 48.1±2.5 | 68.6±4.2 | 56.7±0.2 | 37.4±0.7 | 23.2±4.8 | |||
| F13 | 3,4-(CH3O)2C6H3 | 38.5±4.0 | 25.0±1.6 | 66.2±0.4 | 60.4±3.9 | 54.8±1.9 | 42.1±1.3 | |||
| F14 | 4-BrC6H4 | 73.6±6.1 | 65.8±2.7 | 62.2±0.7 | 54.9±0.4 | 50.2±0.1 | 47.1±7.2 | |||
| F15 | 2,4-Cl2C6H3 | 56.7±2.4 | 45.7±4.9 | 61.7±0.4 | 54.9±0.6 | 55.9±1.1 | 42.4±0.6 | |||
| F16 | 4-CH3C6H4 | 77.4±4.0 | 68.7±1.4 | 53.0±1.9 | 44.6±0.6 | 51.0±1.8 | 33.9±2.3 | |||
| Bismerthiazolb | — | 59.5±7.7 | 49.5±5.7 | 56.0±1.2 | 33.8±2.0 | 61.1±6.2 | 49.5±5.3 | |||
| Compound | R | Toxic regression equation | r | EC50/(μg?mL–1) |
|---|---|---|---|---|
| F4 | 4-O2NC6H4 | y=0.7252x+4.1219 | 0.9871 | 16.3±2.5 |
| F5 | 4-ClC6H4 | y=0.9076x+3.6020 | 0.9559 | 34.7±2.3 |
| F6 | 4-FC6H4 | y=0.9529x+4.0511 | 0.9581 | 9.9±2.1 |
| F7 | 2,4-(CH3O)2C6H3 | y=0.6956x+4.1554 | 0.9889 | 16.4±1.0 |
| F8 | 3-O2NC6H4 | y=0.9427x+3.5545 | 0.9965 | 34.2±2.4 |
| F9 | 2-ClC6H4 | y=0.8344x+3.9314 | 0.9978 | 19.1±2.7 |
| F10 | 4-CH3OC6H4 | y=0.7527x+4.0683 | 0.9897 | 17.3±2.8 |
| F14 | 4-BrC6H4 | y=0.8865x+3.8683 | 0.9899 | 18.9±2.8 |
| F16 | 4-CH3C6H4 | y=0.9412x+3.8669 | 0.9965 | 15.9±1.9 |
| Bismerthiazola | — | y=0.8357x+3.5466 | 0.9876 | 54.9±1.4 |
| Compound | R | Toxic regression equation | r | EC50/(μg?mL–1) |
|---|---|---|---|---|
| F4 | 4-O2NC6H4 | y=0.7252x+4.1219 | 0.9871 | 16.3±2.5 |
| F5 | 4-ClC6H4 | y=0.9076x+3.6020 | 0.9559 | 34.7±2.3 |
| F6 | 4-FC6H4 | y=0.9529x+4.0511 | 0.9581 | 9.9±2.1 |
| F7 | 2,4-(CH3O)2C6H3 | y=0.6956x+4.1554 | 0.9889 | 16.4±1.0 |
| F8 | 3-O2NC6H4 | y=0.9427x+3.5545 | 0.9965 | 34.2±2.4 |
| F9 | 2-ClC6H4 | y=0.8344x+3.9314 | 0.9978 | 19.1±2.7 |
| F10 | 4-CH3OC6H4 | y=0.7527x+4.0683 | 0.9897 | 17.3±2.8 |
| F14 | 4-BrC6H4 | y=0.8865x+3.8683 | 0.9899 | 18.9±2.8 |
| F16 | 4-CH3C6H4 | y=0.9412x+3.8669 | 0.9965 | 15.9±1.9 |
| Bismerthiazola | — | y=0.8357x+3.5466 | 0.9876 | 54.9±1.4 |
| Compound | R | Toxic regression equation | r | EC50/(μg?mL–1) |
|---|---|---|---|---|
| F1 | Ph | y=1.0838x+3.9378 | 0.9488 | 9.6±1.9 |
| F5 | 4-ClC6H4 | y=0.7955x+3.7062 | 0.9066 | 42.3±0.5 |
| F7 | 2,4-(CH3O)2C6H3 | y=0.8195x+3.9491 | 0.9250 | 19.2±0.8 |
| F8 | 3-O2NC6H4 | y=0.8219x+3.6715 | 0.9099 | 41.3±1.4 |
| F10 | 4-CH3OC6H4 | y=0.8897x+3.6013 | 0.9524 | 37.3±1.5 |
| F12 | Furan-2-yl | y=0.9935x+3.5123 | 0.9899 | 31.4±2.2 |
| F13 | 3,4-(CH3O)2C6H3 | y=0.9779x+3.5713 | 0.9402 | 28.9±1.4 |
| F14 | 4-BrC6H4 | y=0.8431x+3.6698 | 0.9886 | 37.8±0.8 |
| F15 | 2,4-Cl2C6H3 | y=0.4375x+4.4190 | 0.9756 | 21.3±0.8 |
| Bismerthiazola | — | y=2.8364x+0.5935 | 0.9897 | 69.3±2.0 |
| Compound | R | Toxic regression equation | r | EC50/(μg?mL–1) |
|---|---|---|---|---|
| F1 | Ph | y=1.0838x+3.9378 | 0.9488 | 9.6±1.9 |
| F5 | 4-ClC6H4 | y=0.7955x+3.7062 | 0.9066 | 42.3±0.5 |
| F7 | 2,4-(CH3O)2C6H3 | y=0.8195x+3.9491 | 0.9250 | 19.2±0.8 |
| F8 | 3-O2NC6H4 | y=0.8219x+3.6715 | 0.9099 | 41.3±1.4 |
| F10 | 4-CH3OC6H4 | y=0.8897x+3.6013 | 0.9524 | 37.3±1.5 |
| F12 | Furan-2-yl | y=0.9935x+3.5123 | 0.9899 | 31.4±2.2 |
| F13 | 3,4-(CH3O)2C6H3 | y=0.9779x+3.5713 | 0.9402 | 28.9±1.4 |
| F14 | 4-BrC6H4 | y=0.8431x+3.6698 | 0.9886 | 37.8±0.8 |
| F15 | 2,4-Cl2C6H3 | y=0.4375x+4.4190 | 0.9756 | 21.3±0.8 |
| Bismerthiazola | — | y=2.8364x+0.5935 | 0.9897 | 69.3±2.0 |
| Compound | R | Toxic regression equation | r | EC50/(μg?mL–1) |
|---|---|---|---|---|
| F3 | 3,4-(CH3)2C6H3 | y=0.7267x+4.1628 | 0.9547 | 14.2±3.3 |
| F6 | 4-FC6H4 | y=0.8243x+4.0431 | 0.9917 | 14.5±4.9 |
| Bismerthiazola | — | y=0.9991x+3.2599 | 0.9399 | 82.6±0.9 |
| Compound | R | Toxic regression equation | r | EC50/(μg?mL–1) |
|---|---|---|---|---|
| F3 | 3,4-(CH3)2C6H3 | y=0.7267x+4.1628 | 0.9547 | 14.2±3.3 |
| F6 | 4-FC6H4 | y=0.8243x+4.0431 | 0.9917 | 14.5±4.9 |
| Bismerthiazola | — | y=0.9991x+3.2599 | 0.9399 | 82.6±0.9 |
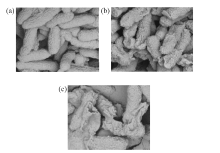
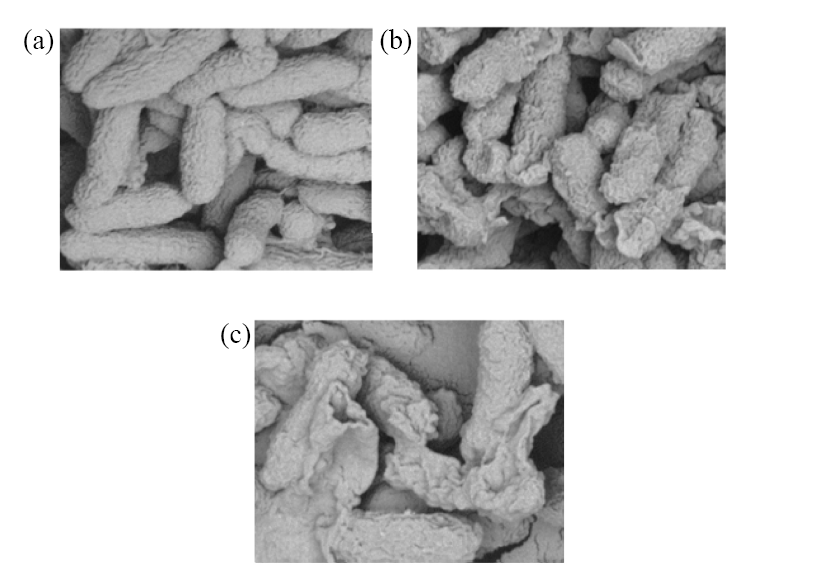
| [1] | Chen, L.-J.; Li, P.; Wang, X.-B.; Ruan, X.-H.; Xue, W. Chem. Bull. 2017, 80, 1156(in Chinese). |
| ( 陈丽娟, 李普, 王晓斌, 阮祥辉, 薛伟, 化学通报, 2017, 80, 1156.) | |
| [2] |
Ren, Y. H.; Jin, H.; Tao, K.; Hou, T. P . Mol. Cell. Toxicol. 2015, 11, 187.
doi: 10.1007/s13273-015-0017-3 |
| [3] | Peron, F.; Lazarin-Bidóia, D.; Din, Z. U.; Rodrigues-Filho, E.; Ueda-Nakamura, T.; Silva, S. O.; Nakamura.V, C. Biomed. Res. Int. 2017, 1. |
| [4] |
Wu, J.; Zhu, Y. Y.; Zhao, Y. H.; Shan, W. L.; Hu, D. Y.; Chen, J.X. Chin. Chem. Lett. 2016, 27, 948.
doi: 10.1016/j.cclet.2016.01.051 |
| [5] |
Chen, C. L.; Chen, J.; Gu, H. Y.; Bao, N.; Dai, H. Molecules 2017, 22, 1126.
doi: 10.3390/molecules22071126 |
| [6] |
Zhou, J.; Tao, Q. Q.; Wang, P. Y.; Shao, W. B.; Wu, Z. B.; Li, Z. Bioorg. Med. Chem. Lett. 2018, 28, 1742.
doi: 10.1016/j.bmcl.2018.04.034 |
| [7] |
Zhu, H. P.; Xu, T. T.; Qiu, C. Y.; Wu, B. B.; Zhang, Y. L.; Chen, L.F. Eur. J. Med. Chem. 2016, 121, 181.
doi: 10.1016/j.ejmech.2016.05.041 |
| [8] |
Wang, Z. S.; Chen, L. Z.; Zhou, H. P.; Liu, X. H; Chen, F.H. . Bioorg. Med. Chem. Lett. 2017, 27, 1803.
doi: 10.1016/j.bmcl.2017.02.056 |
| [9] |
Luo, H.; Yang, S. J.; Cai, Y. Q.; Peng, Z. J.; Liu, T. Eur. J. Med. Chem. 2014, 84, 746.
doi: 10.1016/j.ejmech.2014.07.053 |
| [10] |
Badr, G.; Gul, H. I.; Yamali, C.; Mohamed, A. A. M.; Badr, B. M.; Gul, M. . Bioorg. Chem. 2018, 78, 46.
doi: 10.1016/j.bioorg.2018.03.006 |
| [11] |
Luo, H.; Yang, S. J.; Hong, D.; Xue, W.; Xie, P. Chem. Cent. J. 2017, 11, 23.
doi: 10.1186/s13065-017-0253-9 |
| [12] | Xue, W.; Gong, H.-Y.; Qiu, Q.-J.; Zhao, H.-J.; Li, H.-C.; Han, F.-F. Chem. Reag. 2013, 35, 201 (in Chinese). |
| ( 薛伟, 龚华玉, 仇秋娟, 赵洪菊, 李海畅, 韩菲菲, 化学试剂, 2013, 35, 201.) | |
| [13] | Zhang, J.-P.; Li, P.; Wang, Y.-H.; Zhang, C.; Chen, L.-J.; Tang, X.; He, M.; Xue, W . Chem. J. Chin. Univ 2018, 39, 1455(in Chinese). |
| ( 张菊平, 李普, 王一会, 张橙, 陈丽娟, 汤旭, 贺鸣, 薛伟 , 高等学校化学学报, 2018, 39, 1455.) | |
| [14] | Kulabaş, N.; Tatar, E.; Özakpınar, Ö. B.; Özsavcı, D.; Pannecouque, C.; Clercq, E. D Küçükgüzel, İ. .; Eur. J. Med. Chem. 2016, 121, 58. |
| [15] |
Somagond, S. M.; Kamble, R. R.; Kattimani, P. P.; Shaikh, S. K. J.; Dixit, S. R.; Joshi, S.D. ChemistrySelect 2018, 3, 2004.
doi: 10.1002/slct.201702279 |
| [16] |
Wang, X. B.; Zhong, X. M.; Zhu, X. S.; Wang, H.; Li, Q.; Zhang, J. P.; Xue, W . Chem. Pap. 2017, 71, 1953.
doi: 10.1007/s11696-017-0189-5 |
| [17] |
Zhai, Z.-W.; Wang, Q.; Shen, Z.-H.; Tan, C.-X.; Weng, J.-Q Liu, X.-H. .; Chin. J. Org. Chem. 2017, 37, 232(in Chinese).
doi: 10.6023/cjoc201607031 |
|
( 翟志文, 汪乔, 沈钟华, 谭成侠, 翁建全, 刘幸海, 有机化学 , 2017, 37, 232.)
doi: 10.6023/cjoc201607031 |
|
| [18] |
Başaran, E.; Karaküçük-Iyidoğan, A.; Schols, D.; Oruç-Emre, E. E. Chirality 2016, 28, 495.
doi: 10.1002/chir.v28.6 |
| [19] | Jin, R. Y.; Liu, J. L.; Zhang, G. H.; Li, J. J.; Zhang, S.; Guo, H . Chem. Biodiversity 2018. |
| [20] |
Xu, F. Z.; Shao, J. H.; Zhu, Y. Y.; Liu, L. W.; Zhao, Y. H.; Shan, W. L . Chem. Pap. 2017, 71, 729.
doi: 10.1007/s11696-016-0006-6 |
| [21] |
Du, H.; Fan, Z.-J.; Yang, L.; Bao, X.-P. Chin. J. Org. Chem. 2018, 38, 531 (in Chinese).
doi: 10.6023/cjoc201708051 |
|
( 杜欢, 范治江, 杨岚, 鲍小平, 有机化学 , 2018, 38, 531.)
doi: 10.6023/cjoc201708051 |
|
| [22] |
Yan, B.-R.; Lü, X.-Y.; Du, H.; Bao, X.-P. Chin. J. Org. Chem. 2016, 36, 207(in Chinese).
doi: 10.6023/cjoc201506026 |
|
( 闫柏任, 吕新阳, 杜欢, 鲍小平, 有机化学, 2016, 36, 207.)
doi: 10.6023/cjoc201506026 |
|
| [23] |
Lin, G.-S.; Chen, Z.-C.; Duan, W.-G.; Wang, X.-Y.; Lei, F.-H . Chin. J. Org. Chem. 2018, 38, 2085(in Chinese).
doi: 10.6023/cjoc201801043 |
|
( 林桂汕, 陈智聪, 段文贵, 王晓宇, 雷福厚, 有机化学, 2018, 38, 2085.)
doi: 10.6023/cjoc201801043 |
|
| [24] | Zhou, J.M.S. . Thesis, Guizhou University,Guiyang , 2018 (in Chinese). |
| ( 周建, 硕士论文, 贵州大学 , 贵阳, 2018.) | |
| [25] | Liu, C.-Y.; Zhao, Q.-Q.; Li, J . Chem. Reag. 2001, 23, 344(in Chinese). |
| ( 柳翠英, 赵全芹, 李娟, 化学试剂, 2001, 23, 344.) | |
| [26] |
Gan, X. H.; Hu, D. Y.; Li, P.; Wu, J.; Chen, X. W.; Xue, W.; Song, B.A. Pest Manage. Sci. 2015, 72, 534.
doi: 10.1002/ps.2016.72.issue-3 |
| [27] |
Wu, F.; Li, P.; Hu, D. Y.; Song, B.A. Res. Chem. Intermed. 2016, 42, 7153.
doi: 10.1007/s11164-016-2524-9 |
| [28] |
Xu, W. M.; Han, F. F.; He, M.; Hu, D. Y.; He, J.; Yang, S.; Song, B.A. J. Agric. Food Chem. 2012, 60, 1036.
doi: 10.1021/jf203772d |
| [1] | 光明甲, 姜硕, 朱宝玉, 张如松, 王鲲鹏, 王明慧, 许良忠. 新型吡咯-2-甲酸及其衍生物的设计、合成和杀虫、杀螨活性[J]. 有机化学, 2023, 43(8): 2895-2904. |
| [2] | 何金燕, 田富云, 吴青青, 郑月明, 陈玉婷, 许海燕, 金正盛, 詹丽, 程新强, 顾跃玲, 高召兵, 赵桂龙. 基于[3.3.3]螺桨烷的电压门控钙离子通道α2δ亚基配体的合成和生物活性研究[J]. 有机化学, 2023, 43(6): 2226-2238. |
| [3] | 孙洋, 王杨, 张紫婵, 钱烨, 骆桂成, 周贝贝, 缪丽沙, 陈雨蝶, 戴红, 徐宝琳, 吴正光. 新型含1,3,4-噁二唑基团的吡唑肟衍生物的合成与生物活性[J]. 有机化学, 2023, 43(4): 1584-1590. |
| [4] | 王启帆, 张源泉, 幸丽, 周远香, 龚晨裕, 何帮灿, 张念, 吴拥军, 薛伟. 含1,2,4-三唑并[3,4-b]-1,3,4-噻二唑杨梅素衍生物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(4): 1525-1536. |
| [5] | 雍灿, 李芸, 毕涛, 陈国凤, 郑东霞, 王周玉, 张园园. 基于D-半乳糖衍生的小分子半乳糖凝集素抑制剂的合成及活性研究进展[J]. 有机化学, 2022, 42(5): 1307-1325. |
| [6] | 罗洁, 颜雅倩, 王浩鑫, 李瑶瑶. 新多环大环内酰胺Clifednamide K的发现[J]. 有机化学, 2022, 42(4): 1224-1228. |
| [7] | 毛雅君, 邵香敏, 李阳杰, 曹瑞梅, 冯亚莉, 翟广玉. 槲皮素衍生物的合成研究进展[J]. 有机化学, 2022, 42(11): 3588-3605. |
| [8] | 周莎, 王目阔, 谢伟彬, 周沙, 熊丽霞, 赵毓, 李正名. 新型含N-COCF3硫亚胺取代的手性双酰胺类化合物的合成与生物活性研究[J]. 有机化学, 2021, 41(9): 3532-3538. |
| [9] | 章乐天, 苏嘉媛, 徐晓勇. 作为杀菌和杀虫先导的1,2,4-噁二嗪衍生物的设计与合成研究[J]. 有机化学, 2021, 41(9): 3539-3549. |
| [10] | 邵继炎, 李志杰, 王迓君, 熊裕焱, 胡向东. 环庚三烯酮衍生物的合成和抑制胃癌细胞增殖的活性研究[J]. 有机化学, 2021, 41(9): 3675-3681. |
| [11] | 朱海梦, 王超, 宗利利. 亚砜化合物的生物活性研究和不对称合成进展[J]. 有机化学, 2021, 41(9): 3431-3447. |
| [12] | 徐洪亮, 苏静, 王子时, 侯晨忻, 吴鹏冲, 邢月, 李香帅, 朱晓磊, 路运才, 徐利剑. 苯基吡咯类杀菌剂的设计合成及三维-定量构效关系(3D-QSAR)研究[J]. 有机化学, 2021, 41(9): 3560-3570. |
| [13] | 江婷, 蒲洪, 段燕文, 颜晓晖, 黄勇. 深海、沙漠、火山、极地来源链霉菌新天然产物(2009~2020)[J]. 有机化学, 2021, 41(5): 1804-1820. |
| [14] | 秦啸天, 张俊朝, 何钰晴, 张锐, 程华, 陈宬, 秦鑫. 含4-氨基二芳醚结构的辅酶Q衍生物的合成与生物活性研究[J]. 有机化学, 2021, 41(5): 2045-2054. |
| [15] | 许萌, 高燊原, 曾源煦, 高安慧, 高立信, 许磊, 周宇波, 高建荣, 叶青, 李佳. 3-(吲哚-3-基)-4-(吡唑并[3,4-c]哒嗪-3-基)马来酰亚胺脱氢类异柠檬酸酶-1突变体高效抑制剂的合成与评价[J]. 有机化学, 2021, 41(5): 1991-2000. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||