

基于简单高效钯纳米粒子催化Suzuki偶联反应的研究
收稿日期: 2019-04-27
网络出版日期: 2019-07-03
基金资助
国家自然科学基金(21602144);四川省科技计划(2018JY0485);西华师范大学科研启动基金(17E049)
An Efficient Palladium Nanoparticles Catalytic System for Suzuki Coupling Reactions
Received date: 2019-04-27
Online published: 2019-07-03
Supported by
the National Natural Science Foundation of China(21602144);the Sichuan Science and Technology Program(2018JY0485);the Fundamental Research Funds of China West Normal University(17E049)
李恒超 , 赵玲 , 刘燕 , 张霞 , 李王兵 , 敬林海 , 黄锦 , 汪伟 . 基于简单高效钯纳米粒子催化Suzuki偶联反应的研究[J]. 有机化学, 2019 , 39(11) : 3207 -3214 . DOI: 10.6023/cjoc201904069
A simple and highly efficient palladium nanoparticles catalytic system was applied in Suzuki coupling reaction. This system could catalyze a variety of aryl halide and arylboronic acid substrates with a wide range of functional groups. A high turnover number of 90000 was obtained with the catalyst loading as low as 0.001 mol%. This catalyst system exhibited good stability and longevity.
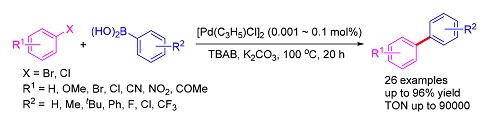
Key words: aryl hailde; arylboronic acid; palladium nanoparticle; Suzuki coupling
| [1] | Miyaura N.; Suzuki A. Chem. Rev. 1995, 95 2457. |
| [2] | Suzuki A. Chem. Commun. 2005 4759. |
| [3] | Suzuki A. Angew. Chem., Int. Ed. 2011, 50 6722. |
| [4] | Capdeville R.; Buchdunger E.; Zimmermann J.; Matter A. Nat. Rev. Drug Discovery 2002, 1 493. |
| [5] | Corbet J. P.; Mignani G. Chem. Rev. 2006, 106 2651. |
| [6] | Magano J.; Dunetz J. R. Chem. Rev. 2011, 111 2177. |
| [7] | Fihri A.; Bouhrara M.; Nekoueishahraki B.; Basset J.-M.; Polshettiwar V. Chem. Soc. Rev. 2011, 40 5181. |
| [8] | (a) Jana, R.; Pathak, T. P.; Sigman, M. S. Chem. Rev. 2011, 111, 1417. |
| [8] | (b) Stockton, K. P.; Merritt, C. J.; Sumby, C. J.; Greatrex, B. W. Eur. J. Org. Chem. 2015, 6999. |
| [8] | (c) Li, J.-X.; Yang, S.-R.; Wu, W.-Q.; Jiang, H.-F. Eur. J. Org. Chem. 2018, 1284. |
| [9] | Albisson D. A.; Bedford R. B.; Lawrence S. E.; Scully P. N. Chem. Commun. 1998 2095. |
| [10] | Zapf A.; Ehrentraut A.; Beller M. Angew. Chem., Int. Ed. 2000, 39 4153. |
| [11] | Yuan D.; Huynh H. V. Organometallics 2010, 29 6020. |
| [12] | Bianchini C.; Lee H. M.; Meli A.; Oberhauser W.; Vizza F.; Brüggeller P.; Rainer H. B.; Langes C. Chem. Commun. 2000 777. |
| [13] | Nair P.; Anderson G. K.; Rath N. P. Organometallics 2003, 22 1494. |
| [14] | Imamoto T.; Yashio K.; Crépy K. V. L.; Katagiri K.; Takahashi H.; Kouchi M.; Gridnev I. D. Organometallics 2006, 25 908. |
| [15] | Field L. D.; Messerle B. A.; Smernik R. J.; Hambley T. W.; Turner P. Inorg. Chem. 1997, 36 2884. |
| [16] | (a) Laurenti, D.; Feuerstein, M.; Pèpe, G.; Doucet, H.; Santelli, M. J. Org. Chem. 2001, 66, 1633. |
| [16] | (b) Feuerstein, M.; Laurenti, D.; Bougeant, C.; Doucet, H.; Santelli, M. Chem. Commun. 2001, 325. |
| [16] | (c) Feuerstein, M.; Doucet, H.; Santelli, M. Synlett 2001, 9, 1458. |
| [16] | (d) Feuerstein, M.; Doucet, H.; Santelli, M. Tetrahedron Lett. 2001, 42, 6667. |
| [16] | (e) Feuerstein, M.; Doucet, H.; Santelli, M. J. Organomet. Chem. 2003, 687, 327. |
| [17] | Hierso J.-C.; Fihri A.; Amardeil R.; Meunier P.; Doucet H.; Santelli M.; Donnadieu B. Organometallics 2003, 22 4490. |
| [18] | Zaborova E.; Deschamp J.; Guieu S.; Bleriot Y.; Poli G.; Menand M.; Madec D.; Prestat G.; Sollogoub M. Chem. Commun. 2011, 47 9206. |
| [19] | (a) Wang, K.; Yi, T.; Yu, X.-J.; Zheng, X.-L.; Fu, H.-Y.; Chen, H.; Li, R.-X. Appl. Organomet. Chem. 2012, 26, 342. |
| [19] | (b) Wang, K.; Fu, Q.; Zhou, R.; Zheng, X.-L.; Fu, H.-Y.; Chen, H.; Li, R.-X. Appl. Organomet. Chem. 2013, 27, 232. |
| [19] | (c) Wang, K.; Wang, W.; Luo, H.; Zheng, X.-L.; Fu, H.-Y.; Chen, H.; Li, R.-X. Catal. Lett. 2013, 143, 1214. |
| [19] | (d) Guo, F.-C.; Zhou, R.; Jiang, Z.-J.; Wang, W.; Zheng, X.-L.; Fu, H.-Y.; Chen, H.; Li, R.-X. Catal. Commun. 2015, 66, 87. |
| [20] | (a) Bernhardt, E.; Willner, H.; Jonas, V.; Thiel, W.; Aubke, F. Angew. Chem., Int. Ed. 2000, 39, 165. |
| [20] | (b) Sayah, R.; Glegola, K.; Framery, E.; Dufaud, V. Adv. Synth. Catal. 2007, 349, 373. |
| [20] | (c) Gholinejad, M.; Hamed F.; Biji, P. Dalton Trans. 2015, 44, 14293. |
| [20] | (d) Zhang, Q.; Li, J.-H.; Zhao, X. Chin. J. Org. Chem. 2016, 36, 130 (in Chinese). |
| [20] | (张强, 李继航赵鑫, 有机化学, 2016, 36, 130.) |
| [20] | (e) Yang, P.-B.; Ma, R.; Bian, F.-L. ChemCatChem 2016, 8, 3746. |
| [20] | (f) Sharma, S.; Nazir, R.; Pande, S.; Sarkar, B. R. ChemistrySelect 2017, 2, 8745. |
| [20] | (g) Kunfi, A.; May, Z.; Németh, P.; London, G. J. Catal. 2018, 361, 84. |
| [20] | (h) Liu, X.-M.; Tang, B.; Long, J.-L.; Zhang, W.; Liu, X. H.; Mirza, Z. Sci. Bull. 2018, 63, 502. |
| [20] | (i) Fu, Y.-F.; Zou, Z.-J.; Tang, C.; Song, K.-P. Chin. J. Org. Chem. 2018, 38, 3106 (in Chinese). |
| [20] | (付玉芳, 邹志娟, 唐成, 宋昆鹏, 有机化学, 2018, 38, 3106.) |
| [20] | (j) Narkhede, N.; Uttam, B.; Rao, C. P. ACS Omega 2019, 4, 4908. |
| [21] | (a) Wang, W.; Yang, Q.; Zhou, R.; Fu, H.-Y.; Zheng, X.-L.; Chen, H.; Li, R.-X. J. Organomet. Chem. 2012, 697, 1. |
| [21] | (b) Wang, W.; Zhou, R.; Jiang, Z.-J.; Wang, K.; Fu, H.-Y.; Zheng, X.-L.; Chen, H.; Li, R.-X. Adv. Synth. Catal. 2014, 356, 616. |
| [21] | (c) Wang, W.; Zhou, R.; Jiang, Z.-J.; Wang, X.; Fu, H.-Y.; Zheng, X.-L.; Chen, H.; Li, R.-X. Eur. J. Org. Chem. 2015, 2579. |
| [21] | (d) Huang, J.; Fu, R.-H.; Jing, L.-H.; Qin, D.-B.; Huang, K.; Wang, W. Chin. J. Org. Chem. 2019, 39, 456 (in Chinese). |
| [21] | (黄锦, 付荣辉, 敬林海, 秦大斌, 黄昆, 汪伟, 有机化学, 2019, 39, 456.) |
| [21] | (e) He, H.-Y.; Wang, W.; Yu, X.-J.; Huang, J.; Jian, L.; Fu, H.-Y.; Zheng, X.-L.; Chen, H.; Li, R.-X. Eur. J. Org. Chem. 2016, 56169. |
| [22] | Arumugam V.; Kaminsky W.; Bhuvanesh N. S. P.; Nallasamy D. RSC Adv. 2015, 5 59428. |
| [23] | Lysén M.; K?hler K. Synthesis 2006, 4 692. |
| [24] | Jansa J.; Jambor R. Appl. Organomet. Chem. 2016, 30 1036. |
| [25] | Ahmed J.; Chakraborty S.; Jose A.; Mandal S. K. J. Am. Chem. Soc. 2018, 140 8330. |
| [26] | Klein M.; Voigtmann U.; Haack T.; Erdinger L.; Boche G. Mutat. Res. 2000, 467 55. |
| [27] | Macé Y.; Raymondeau B.; Pradet C.; Blazejewski J. C.; Magnier E. Eur. J. Org. Chem. 2009 1390. |
| [28] | Ernst J. B.; Rakers L.; Glorius F. Synthesis 2017, 49 260. |
| [29] | Duan X.-Y.; Li P.-B.; Zhu G.-R.; Fu C.-L.; Chen Q.; Huang X.; Ma S.-M. Org. Chem. Front. 2018, 5 3319. |
| [30] | Chiu C. C.; Chiu H. T.; Lee D. S.; Lu T. J. RSC Adv. 2018, 8 26407. |
/
| 〈 |
|
〉 |