

非光催化与电化学体系下有机硫试剂参与的烯烃构建C—S键研究进展
收稿日期: 2020-06-29
修回日期: 2020-07-24
网络出版日期: 2020-08-11
基金资助
广州市科技计划科学研究专项(201607010251); 广东省科技计划(2017A010103016); 广东省功能分子工程重点实验室开放课题(2017kf01)
Research Progress in C—S Bond Formation Reaction of Olefins with Organic Sulfur Reagents under Photocatalyst-Free and Non-Electrochemical Conditions
Received date: 2020-06-29
Revised date: 2020-07-24
Online published: 2020-08-11
Supported by
the Science and Technology Project Scientific Special of Guangzhou City(201607010251); the Guangdong Provincial Science and Technology Project(2017A010103016); the Open Fund of the Key Laboratory of Functional Molecular Engineering of Guangdong Province(2017kf01)
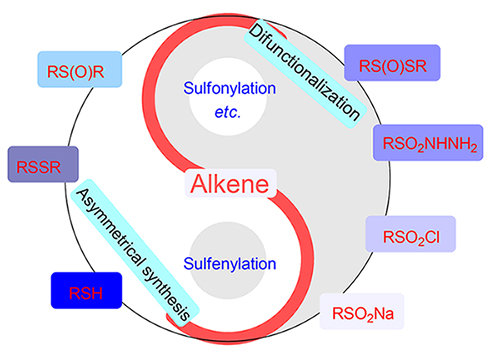
烯烃是重要的合成子, 其简洁高效的合成转化一直是有机合成领域的研究热点. 近年来, 基于烯烃骨架的C—S键成键反应特别受科学家的关注. 烯烃可以与常见的有机含硫试剂(包括硫醇或硫酚、二硫醚、亚砜、磺酰肼、磺酰氯和亚磺酸钠等)反应, 在烯烃的 α-位或者 β-位构筑C—S键来合成硫醚、亚砜或砜类化合物. 鉴于此, 以有机硫试剂的种类为分类依据, 综述了近年来非光催化与电化学体系下有机硫试剂参与的烯烃构建C—S反应的相关研究. 展望未来, 在烯烃与有机硫试剂的C—S成键反应研究中, 双官能团化反应和不对称催化合成具有较好的应用前景.
王柏文 , 周永军 , 罗时荷 , 罗晓燕 , 陈伟清 , 杨诗敏 , 汪朝阳 . 非光催化与电化学体系下有机硫试剂参与的烯烃构建C—S键研究进展[J]. 有机化学, 2021 , 41(1) : 171 -184 . DOI: 10.6023/cjoc202006064
Olefin is an important synthetic synthon, and its concise and efficient transformation has been a research hotspot in the field of organic synthesis. In recent years, the C—S bond formation based on the olefin skeleton has received particular attention from scientists. Alkenes can react with common organic sulfur-containing reagents, including thiol, disulfide, sulfoxide, sulfonyl hydrazide, sulfonyl chloride, sodium sulfinate and other kinds of organosulfur reagents to build C—S bonds at the α-position or β-position of olefin to synthesize sulfide, sulfoxide or sulfone. Thus, according to the different classification of the used organosulfur reagents, the recent research progresses in C—S bond formation reaction of olefins with organic sulfur reagents under photocatalyst-free conditions and non-electrochemical method is summarized. In the future, among the researches on the C—S bond formation reactions of olefins with inexpensive organosulfur reagents, asymmetrical synthesis and various difunctionalizations are still promising directions.

| [1] | Trost B.M.; Kalnmals C.A. Chem.-Eur. J. 2019, 25, 11193. |
| [2] | Hofman K.; Liu N.-W.; Manolikakes G. Chem.-Eur. J. 2018, 24, 11852. |
| [3] | Mampuys P.; McElroy C.R.; Clark J.H.; Orru R.V.A.; Maes B.U.W. Adv. Synth. Catal. 2020, 362, 3. |
| [4] | Zhang S.N.; Yang S.H.; Huang L.H.; Zhao B.L.; Cheng K.; Qi C.Z. Chin. J. Org. Chem. 2015, 35, 2259. (in Chinese) |
| [4] | ( 张诗浓, 杨胜虎, 黄乐浩, 赵保丽, 程凯, 齐陈泽, 有机化学, 2015, 35, 2259.). |
| [5] | Fang, Y.Y; Luo, Z.G.; Xu, X.M. RSC Adv. 2016, 6, 59661. |
| [6] | Huang G.B.; Li X.Y.; Luo J.R.; Luo Z.H.; Tan M.X. Chin. J. Org. Chem. 2019, 39, 617. (in Chinese) |
| [6] | ( 黄国保, 李秀英, 罗金荣, 罗志辉, 谭明雄, 有机化学, 2019, 39, 617.). |
| [7] | Guo W.; Tao K.L.; Tan W.; Zhao M.M.; Zheng L.Y.; Fan X.L. Org. Chem. Front. 2019, 6, 2048. |
| [8] | Li Y.Q.; Fan Y.H. Synth. Commun. 2019, 49, 3227. |
| [9] | Cabrero-Antonino J.R.; Leyva-Perez A.; Corma A. Adv. Synth. Catal. 2012, 354, 678. |
| [10] | Mosaferi E.; Ripsman D.; Stephan D.W. Chem. Commun. 2016, 52, 8291. |
| [11] | Kucinski K.; Pawluc P.; Hreczycho G. Adv. Synth. Catal. 2015, 357, 3936. |
| [12] | Taniguchi N. ChemistrySelect 2018, 3, 6209. |
| [13] | Taniguchi N.; Kitayama K. Synlett 2018, 29, 2712. |
| [14] | Chun S.; Chung J.; Park J.E.; Chung Y.K. ChemCatChem 2016, 8, 2476. |
| [15] | Rosa C.H.; Peixoto M.L.B.; Rosa G.R.; Godoi B.; Galetto F.Z.; D'Oca M.G.M.; Godoi M. Tetrahedron Lett. 2017, 58, 3777. |
| [16] | Biermann U.; Metzger J.O. Eur. J. Org. Chem. 2018, 730. |
| [17] | Choudhuri K.; Mandal A.; Mal P. Chem. Commun. 2018, 54, 3759. |
| [18] | Lu Q.Q.; Wang H.M.; Peng P.; Liu C.; Huang Z.Y.; Luo Y.; Lei A.W. Org. Chem. Front. 2015, 2, 908. |
| [19] | Wang H.M.; Wang G.Y.; Lu Q.Q.; Chiang C.-W.; Peng P.; Zhou J.F.; Lei A.W. Chem.-Eur. J. 2016, 22, 14489. |
| [20] | Li C.S.; Li J.X.; Tan C.W.; Wu W.Q.; Jiang H.F. Org. Chem. Front. 2018, 5, 3158. |
| [21] | Zheng Y.; He Y.; Rong G.W.; Zhang X.L.; Weng Y.C.; Dong K.Y.; Xu X.F.; Mao J.C. Org. Lett. 2015, 17, 5444. |
| [22] | Cui H.H.; Liu X.X.; Wei W.; Yang D.S.; He C.L.; Zhang T.T.; Wang H. J. Org. Chem. 2016, 81, 2252. |
| [23] | Wang D.Y.; Yan Z.H.; Xie Q.H.; Zhang R.X.; Lin S.; Wang Y.X. Org. Biomol. Chem. 2017, 15, 1998. |
| [24] | Liu S.S.; Klussmann M. Chem. Commun. 2020, 56, 1557. |
| [25] | Zhou S.-F.; Pan X.Q.; Zhou Z.-H.; Shoberu A.; Zou J.-P. J. Org. Chem. 2015, 80, 3682. |
| [26] | Du B.N.; Wang Y.; Mei H.B.; Han J.L.; Pan Y. Adv. Synth. Catal. 2017, 359, 1684. |
| [27] | Zhao J.-J.; Tang M.; Zhang H.-H.; Xu M.-M.; Shi F. Chem. Commun. 2016, 52, 5953. |
| [28] | Hui Y.H.; Jiang J.; Wang W.T.; Chen W.L.; Cai Y.F.; Lin L.L.; Liu X.H.; Feng X.M. Angew. Chem., Int. Ed. 2010, 49, 4290. |
| [29] | Tian X.; Cassani C.; Liu Y.K.; Moran A.; Urakawa A.; Galzerano P.; Arceo E.; Melchiorre P. J. Am. Chem. Soc. 2011, 133, 17934. |
| [30] | Kitanosono T.; Sakai M.; Ueno M.; Kobayashi S. Org. Biomol. Chem. 2012, 10, 7134. |
| [31] | White J.D.; Shaw S. Chem. Sci. 2014, 5, 2200. |
| [32] | Chen J.A.; Meng S.X.; Wang L.M.; Tang H.M.; Huang Y. Chem. Sci. 2015, 6, 4184. |
| [33] | Farley A.J.M.; Sandford C.; Dixon D.J. J. Am. Chem. Soc. 2015, 137, 15992. |
| [34] | Huo C.D.; Wang Y.J.; Yuan Y.; Chen F.J.; Tang J. Chem. Commun. 2016, 52, 7233. |
| [35] | Xi H.; Deng B.C.; Zong Z.Z.; Lu S.L.; Li Z.P. Org. Lett. 2015, 17, 1180. |
| [36] | He L.; Guo H.; Li Y.-Z.; Du G.-F.; Dai B. Chem. Commun. 2014, 50, 3719. |
| [37] | Cong Z.-S.; Li Y.-G.; Du G.-F.; Gu C.-Z.; Dai B.; He L. Chem. Commun. 2017, 53, 13129. |
| [38] | Kawazoe S.; Yoshida K.; Shimazaki Y.; Oriyama T. Tetrahedron Lett. 2015, 56, 410. |
| [39] | Li Z.; Song G.Y.; He J.J.; Du Y.; Yang J.Y. J. Sulfur Chem. 2017, 38, 686. |
| [40] | Wei Q.; Hou W.D.; Liao N.; Peng Y.G. Adv. Synth. Catal. 2017, 359, 2364. |
| [41] | Wan J.-P.; Zhong S.S.; Xie L.L.; Cao X.J.; Liu Y.Y.; Wei L. Org. Lett. 2016, 18, 584. |
| [42] | Jiang Y.J.; Liang G.H.; Zhang C.; Loh T.-P. Eur. J. Org. Chem. 2016, 3326. |
| [43] | Siddaraju Y.; Prabhu K.R. J. Org. Chem. 2017, 82, 3084. |
| [44] | Orsi D.L.; Easley B.J.; Lick A.M.; Altman R.A. Org. Lett. 2017, 19, 1570. |
| [45] | Tamai T.; Ogawa A. J. Org. Chem. 2014, 79, 5028. |
| [46] | Tamai T.; Fujiwara K.; Higashimae S.; Nomoto A.; Ogawa A. Org. Lett. 2016, 18, 2114. |
| [47] | Yang X.-H.; Davison R.T.; Nie S.-Z.; Cruz F.A.; McGinnis T.M.; Dong V.M. J. Am. Chem. Soc. 2019, 141, 3006. |
| [48] | Yang X.-H.; Davison R.T.; Dong V.M. J. Am. Chem. Soc. 2018, 140, 10443. |
| [49] | Zhang Y.X.; Wong Z.R.; Wu X.X.; Lauw S.J.L.; Huang X.; Webster R.D.; Chi Y.G.R. Chem. Commun. 2016, 53, 184. |
| [50] | Wang L.L.; Yue H.L.; Yang D.S.; Cui H.H.; Zhu M.H.; Wang J.M.; Wei W.; Wang H. J. Org. Chem. 2017, 82, 6857. |
| [51] | Liang X.; Xiong M.T.; Zhu H.P.; Shen K.X.; Pan Y.J. J. Org. Chem. 2019, 84, 11210. |
| [52] | Wang Y.J.; Jiang W.; Huo C.D. J. Org. Chem. 2017, 82, 10628. |
| [53] | Singh A.K.; Chawla R.; Keshari T.; Yadav V.K.; Yadav L.D.S. Org. Biomol. Chem. 2014, 12, 8550. |
| [54] | Yang L.; Wen Q.; Xiao F.H.; Deng G.-J. Org. Biomol. Chem. 2014, 12, 9519. |
| [55] | Tu H.-Y.; Hu B.-L.; Deng C.-L.; Zhang X.-G. Chem. Commun. 2015, 51, 15558. |
| [56] | Liu Y.R.; Hu B.L.; Zhang X.G. Chin. J. Chem. 2017, 35, 307. |
| [57] | Sun K.; Lv Y.H.; Shi Z.D.; Mu S.Q.; Li C.H.; Wang X. Org. Biomol. Chem. 2017, 15, 5258. |
| [58] | Liu C.; Fang Y.; Wang S.-Y.; Ji S.-J. ACS Catal. 2019, 9, 8910. |
| [59] | Lin C.; Li D.Y.; Wang B.J.; Yao J.Z.; Zhang Y.H. Org. Lett. 2015, 17, 1328. |
| [60] | Liu S.-L.; Li X.-H.; Shi T.-H.; Yang G.-C.; Wang H.-L.; Gong J.-F.; Song M.-P. Eur. J. Org. Chem. 2017, 2280. |
| [61] | Shang Z.H.; Chen Q.Y.; Xing L.L.; Zhang Y.L.; Wait L.; Du Y.F. Adv. Synth. Catal. 2019, 361, 4926. |
| [62] | Liu C.; Fang Y.; Wang S.-Y.; Ji S.-J. Org. Lett. 2018, 20, 6112. |
| [63] | Tan Y.-H.; Li J.-X.; Hong W.-K.; Wang Z.-Y. Chin. J. Org. Chem. 2011, 31, 616. (in Chinese) |
| [63] | ( 谭越河, 李建晓, 洪文坤, 汪朝阳, 有机化学, 2011, 31, 616.). |
| [64] | Mao C.-X.; Wang Z.-Y.; Tan Y.-H.; Xue F.-L. Chin. J. Org. Chem. 2011, 31, 1377. (in Chinese) |
| [64] | ( 毛超旭, 汪朝阳, 谭越河, 薛福玲, 有机化学, 2011, 31, 1377.). |
| [65] | Liu J.; Chen W.; Wang L. RSC Adv. 2013, 3, 4723. |
| [66] | Cao L.; Luo S.-H.; Jiang K.; Hao Z.-F.; Wang B.-W.; Pang C.-M.; Wang Z.-Y. Org. Lett. 2018, 20, 4754. |
| [67] | Li J.; Rao W.D.; Wang S.-Y.; Ji S.-J. J. Org. Chem. 2019, 84, 11542. |
| [68] | Reddy R.J.; Kumar J.J.; Kumari A.H. Eur. J. Org. Chem. 2019, 3771. |
| [69] | Kong W.G.; Yu C.J.; An H.J.; Song Q.L. Org. Lett. 2018, 20, 4975. |
| [70] | Shyam P.K.; Son S.; Jang H.-Y. Eur. J. Org. Chem. 2017, 5025. |
| [71] | Son S.; Shyam P.K.; Park H.; Jeong I.; Jang H.-Y. Eur. J. Org. Chem. 2018, 3365. |
| [72] | Reddy R.J.; Kumari A.H.; Kumar J.J.; Nanubolu J.B. Adv. Synth. Catal. 2019, 361, 1587. |
| [73] | Shukla G.; Srivastava A.; Nagaraju A.; Raghuvanshi K.; Singh M.S. Adv. Synth. Catal. 2015, 357, 3969. |
| [74] | Wang B.-W.; Liu Y.; Hao Z.-F.; Hou J.-Q.; Li J.-Y.; Li S.-T.; Pan S.-H.; Zeng M.-H.; Wang Z.-Y. Chin. J. Org. Chem. 2018, 38, 1872. (in Chinese) |
| [74] | ( 王柏文, 刘园, 郝志峰, 侯佳琦, 李健怡, 李舒婷, 潘思慧, 曾铭豪, 汪朝阳, 有机化学, 2018, 38, 1872.). |
| [75] | Yang K.; Gao J.-J.; Luo S.-H.; Wu H.-Q.; Pang C.-M.; Wang B.-W.; Chen X.-Y.; Wang Z.-Y. RSC Adv. 2019, 9, 19917. |
| [76] | Wu H.-Q.; Yang K.; Chen X.-Y.; Arulkumar M.; Wang N.; Chen S.-H.; Wang Z.-Y. Green Chem. 2019, 21, 3782. |
| [77] | Cao L.; Luo S.-H.; Wu H.-Q.; Chen L.-Q.; Jiang K.; Hao Z.-F.; Wang Z.-Y. Adv. Synth. Catal. 2017, 359, 2961. |
| [78] | Gao X.F.; Pan X.J.; Gao J.; Jiang H.F.; Yuan G.Q.; Li Y.W. Org. Lett. 2015, 17, 1038. |
| [79] | Hua J.W.; Xu J.Q.; Xu J.; Zhou B.C.; Zhang D.; Yang Z.; Fang Z.; Guo K. Eur. J. Org. Chem. 2019, 4056. |
| [80] | He R.F.; Chen X.W.; Li Y.B.; Liu Q.; Liao C.S.; Chen L.; Huang Y.B. J. Org. Chem. 2019, 84, 8750. |
| [81] | Ebule R.; Hammond G.B.; Xu B. Eur. J. Org. Chem. 2018, 4705. |
| [82] | Lan L.Y.; Gao Y.; Ding R.; Zhang T.; Liu Y.G.; Sun B.G.; Tian H.Y. Synth. Commun. 2019, 49, 539. |
| [83] | Gao X.F.; Pan X.J.; Gao J.; Huang H.W.; Yuan G.Q.; Li Y.W. Chem. Commun. 2015, 51, 210. |
| [84] | Jiang Y.J.; Loh T.-P. Chem. Sci. 2014, 5, 4939. |
| [85] | Shao A.L.; Gao M.; Chen S.T.; Wang T.; Lei A.W. Chem. Sci. 2017, 8, 2175. |
| [86] | Huang J.J.; Hu G.; An S.Y.; Chen D.D.; Li M.L.; Li P.F. J. Org. Chem. 2019, 84, 9758. |
| [87] | Yan J.J.; Pulis A.P.; Perry G.J.P.; Procter D.I. Angew. Chem., Int. Ed. 2019, 58, 15675. |
| [88] | Haut F.-L.; Habiger C.; Speck K.; Wurst K.; Mayer P.; Korber J.N.; Muller T.; Magauer T. J. Am. Chem. Soc. 2019, 141, 13352. |
| [89] | Xu X.M.; Li J.Z.; Wang Z.L. Chin. J. Org. Chem. 2020, 40, 886. (in Chinese) |
| [89] | ( 徐鑫明, 李家柱, 王祖利, 有机化学, 2020, 40, 886.). |
| [90] | Deng L.L.; Liu Y.Y. ACS Omega 2018, 3, 11890. |
| [91] | Wang F.-X.; Tian S.-K. J. Org. Chem. 2015, 80, 12697. |
| [92] | Yang F.-L.; Wang F.-X.; Wang T.-T.; Wang Y.-J.; Tian S.-K. Chem. Commun. 2014, 50, 2111. |
| [93] | Yang F.-L.; Gui Y.; Yu B.-K.; Jin Y.-X.; Tian S.-K. Adv. Synth. Catal. 2016, 358, 3368. |
| [94] | Singh R.; Allam B.K.; Singh N.; Kumari K.; Singh S.K.; Singh K.N. Org. Lett. 2015, 17, 2656. |
| [95] | Aegurla B.; Peddinti R.K. Asian J. Org. Chem. 2018, 7, 946. |
| [96] | Liu C.R.; Ding L.H.; Guo G.; Liu W.W. Eur. J. Org. Chem. 2016, 910. |
| [97] | Wei W.; Liu C.L.; Yang D.S.; Wen J.W.; You J.M.; Suo Y.R.; Wang H. Chem. Commun. 2013, 49, 10239. |
| [98] | Wan X.; Sun K.; Zhang G.S. Sci. China: Chem. 2017, 60, 353. |
| [99] | Tang Y.C.; Fan Y.Y.; Gao H.J.; Li X.Q.; Xu X.S. Tetrahedron Lett. 2015, 56, 5616. |
| [100] | Tang Y.C.; Zhang Y.; Wang K.F.; Li X.Q.; Xu X.S.; Du X.H. Org. Biomol. Chem. 2015, 13, 7084. |
| [101] | Chen W.T.; Liu X.Y.; Chen E.; Chen B.H.; Shao J.A.; Yu Y.P. Org. Chem. Front. 2017, 4, 1162. |
| [102] | Terent'ev A.O.; Mulina O.M.; Pirgach D.A.; Demchuk D.V.; Syroeshkin M.A.; Nikishin G.I. RSC Adv. 2016, 6, 93476. |
| [103] | Choudhuri K.; Achar T.K.; Mal P. Adv. Synth. Catal. 2017, 359, 3566. |
| [104] | Sun K.; Lv Y.H.; Zhu Z.H.; Jiang Y.Q.; Qi J.J.; Wu H.K.; Zhang Z.G.; Zhang G.S.; Wang X. RSC Adv. 2015, 5, 50701. |
| [105] | Xu R.; Li Z.P. Tetrahedron Lett. 2018, 59, 3942. |
| [106] | Wang B.; Tang L.; Liu L.Y.; Li Y.N.; Yang Y.; Wang Z.Y. Green Chem. 2017, 19, 5794. |
| [107] | Wang D.Y.; Zhang R.X.; Lin S.; Yan Z.H.; Guo S.M. Synlett 2016, 27, 2003. |
| [108] | Ni J.X.; Jiang Y.; An Z.Y.; Lan J.F.; Yan R.L. Chem. Commun. 2019, 55, 7343. |
| [109] | Jiang L.Q.; Ding T.Q.; Yi W.-B.; Zeng X.; Zhang W. Org. Lett. 2018, 20, 2236. |
| [110] | Wei J.J.; Liang S.S.; Jiang L.Q.; Mumtaz Y.; Yi W.-B. J. Org. Chem. 2020, 85, 977. |
| [111] | Xia Y.Y.; Chen X.L.; Qu L.B.; Sun K.; Xia X.Y.; Fu W.K.; Chen X.; Yang Y.K.; Zhao Y.F.; Li C.J. Asian J. Org. Chem. 2016, 5, 878. |
| [112] | Lai M.; Wu Z.Y.; Li S.-J.; Wei D.H.; Zhao M.Q. J. Org. Chem. 2019, 84, 11135. |
| [113] | Zhu R.; Buchwald S.L. J. Am. Chem. Soc. 2015, 137, 8069. |
| [114] | Shi J.; Tang X.-D.; Wu Y.-C.; Li H.-N.; Song L.-J.; Wang Z.-Y. Eur. J. Org. Chem. 2015, 1193. |
| [115] | Lin Y.-M.; Lu G.-P.; Wang G.-X.; Yi W.-B. J. Org. Chem. 2017, 82, 382. |
| [116] | Wang D.Y.; Zhang R.X.; Ning W.; Yan Z.H.; Lin S. Org. Biomol. Chem. 2016, 14, 5136. |
| [117] | Wang B.-W.; Jiang K.; Li J.-X.; Luo S.-H.; Wang Z.-Y.; Jiang H.-F. Angew. Chem., Int. Ed. 2020, 59, 2338. |
| [118] | Shi J.; Tang X.-D.L; Wu Y.-C.; Fang J.-F.; Cao L.; Chen X.-Y.; Wang Z.-Y. RSC Adv. 2016, 6, 25651. |
| [119] | Zhang N.; Yang D.S.; Wei W.; Yuan L.; Cao Y.J.; Wang H. RSC Adv. 2015, 5, 37013. |
| [120] | Hua L.-N.; Li H.; Qing F.-L.; Huang Y.G.; Xu X.-H. Org. Biomol. Chem. 2016, 14, 8443. |
| [121] | Wu H.-Q.; Luo S.-H.; Cao L.; Shi H.-N.; Wang B.-W.; Wang Z.-Y. Asian J. Org. Chem. 2018, 7, 2479. |
| [122] | Wu H.-Q.; Yang K.; Luo S.-H.; Wu X.-Y.; Wang N.; Chen S.-H.; Wang Z.-Y. Eur. J. Org. Chem. 2019, 28, 4572. |
| [123] | Wu Y.-C.; Luo S.-H.; Mei W.-J.; Cao L.; Wu H.-Q.; Wang Z.-Y. Eur. J. Med. Chem. 2017, 139, 84. |
| [124] | Wu Y.-C.; Cao L.; Mei W.-J.; Wu H.-Q.; Luo S.-H.; Zhan H.-Y.; Wang Z.-Y. Chem. Biol. Drug Des. 2018, 92, 1232. |
| [125] | Luo S.-H.; Yang K.; Lin J.-Y.; Gao J.-J.; Wu X.-Y.; Wang Z.-Y. Org. Biomol. Chem. 2019, 17, 5138. |
| [126] | Cao L.; Li J.-X.; Wu H.-Q.; Jiang K.; Hao Z.-F.; Luo S.-H.; Wang Z.-Y. ACS Sustainable Chem. Eng. 2018, 6, 4147. |
| [127] | Ke M.L.; Huang G.X.; Ding L.; Fang J.J.; Chen F.-E. ChemCatChem 2019, 11, 4720. |
| [128] | Wang L.-J.; Chen M.M.; Qi L.; Xu Z.D.; Li W. Chem. Commun. 2017, 53, 2056. |
| [129] | Kariya A.; Yamaguchi T.; Nobuta T.; Tada N.; Miura T.; Itoh A. RSC Adv. 2014, 4, 13191. |
| [130] | Chawla R.; Singh A.K.; Yadav L.D.S. Eur. J. Org. Chem. 2014, 2032. |
| [131] | Zeng K.; Chen L.; Chen Y.; Liu Y.P.; Zhou Y.B.; Au C.-T.; Yin S.-F. Adv. Synth. Catal. 2017, 359, 841. |
| [132] | Xiong Y.; Sun Y.W.; Zhang G.Z. Org. Lett. 2018, 20, 6250. |
| [133] | Kramer P.; Halaczkiewicz M.; Sun Y.; Kelm H.; Manolikakes G. J. Org. Chem. 2020, 85, 3617. |
| [134] | Gao W.-C.; Cheng Y.-F.; Shang Y.-Z.; Chang H.-H.; Li X.; Zhou R.; Qiao Y.; Wei W.-L. J. Org. Chem. 2018, 83, 11956. |
| [135] | Shen T.; Yuan Y.Z.; Song S.; Jiao N. Chem. Commun. 2014, 50, 4115. |
| [136] | Wei W.; Wen J.W.; Yang D.S.; Du J.; You J.M.; Wang H. Green Chem. 2014, 16, 2988. |
| [137] | Chu X.-Q.; Meng H.; Xu X.-P.; Ji S.-J. Chem.-Eur. J. 2015, 21, 11359. |
| [138] | Wei W.; Wen J.W.; Yang D.S.; Wu M.; You J.M.; Wang H. Org. Biomol. Chem. 2014, 12, 7678. |
| [139] | Lu Q.Q.; Zhang J.; Wei F.L.; Qi Y.; Wang H.M.; Liu Z.L.; Lei A.W. Angew. Chem., Int. Ed. 2013, 52, 7156. |
| [140] | Yuan Z.L.; Wang H.-Y.; Mu X.; Chen P.H.; Guo Y.-L.; Liu G.S. J. Am. Chem. Soc. 2015, 137, 2468. |
| [141] | Liu X.; An R.; Zhang X.L.; Luo J.; Zhao X.D. Angew. Chem., Int. Ed. 2016, 55, 5846. |
| [142] | Luo J.; Liu Y.N.; Zhao X.D. Org. Lett. 2017, 19, 3434. |
| [143] | Zhang J.J.; Yang J.-D.; Zheng H.-L.; Xue X.-S.; Mayr H.; Cheng J.-P. Angew. Chem., Int. Ed. 2018, 57, 12690. |
| [144] | Luo J.; Cao Q.X.; Cao X.H.; Zhao X.D. Nat. Commun. 2018, 9, 527. |
| [145] | Liu X.; Lang Y.Y.; Ji J.Y.; Luo J.; Zhao X. J. Am. Chem. Soc. 2018, 140, 4782. |
| [146] | Zhang P.P.; Li M.; Xue X.-S.; Xu C.-F.; Zhao Q.C.; Liu Y.F.; Wang H.Y.; Guo Y.L.; Lu L.; Shen Q.L. J. Org. Chem. 2016, 81, 7486. |
| [147] | Yoo J.; Ha H.-J.; Kim B.; Cho C.-W. J. Org. Chem. 2020, 85, 7077. |
| [148] | Tian H.; Yu J.P.; Yang H.J.; Zhu C.J.; Fu H. Adv. Synth. Catal. 2016, 358, 1794. |
| [149] | Xiao Q.; He Q.J.; Li J.C.; Wang J. Org. Lett. 2015, 17, 6090. |
| [150] | Jia Y.M.; Qin H.M.; Wang N.; Jiang Z.-X.; Yang Z.G. J. Org. Chem. 2018, 83, 2808. |
| [151] | Sicignano M.; Rodriguez R.I.; Capaccio V.; Borello F.; Cano R.; De Riccardis F.; Bernardi L.; Diaz-Tendero S.; Della Sala G.; Aleman J. Org. Biomol. Chem. 2020, 18, 2914. |
| [152] | Jin M.Y.; Li J.C.; Huang R.K.; Zhou Y.X.; Chung L.W.; Wang J. Chem. Commun. 2018, 54, 4581. |
| [153] | Liang Y.Y.; Zhao X.D. ACS Catal. 2019, 9, 6896. |
| [154] | Xu C.F.; Shen Q.L. Org. Lett. 2015, 17, 4561. |
| [155] | Yang T.; Lu L.; Shen Q.L. Chem. Commun. 2015, 51, 5479. |
| [156] | Luo J.; Zhu Z.C.; Liu Y.N.; Zhao X.D. Org. Lett. 2015, 17, 3620. |
| [157] | Zhu Z.C.; Luo J.; Zhao X.D. Org. Lett. 2017, 19, 4940. |
| [158] | Song T.; Zhao X.M.; Wang X.L. J. Org. Chem. 2019, 84, 15648. |
| [159] | Nguyen T.B. Adv. Synth. Catal. 2020, 362, 3448. |
| [160] | Yin F.; Wang X.-S. Org. Lett. 2014, 16, 1128. |
| [161] | Cheng Z.-F.; Tao T.-T.; Feng Y.-S.; Tang W.-K.; Xu J.; Dai J.-J.; Xu H.-J. J. Org. Chem. 2018, 83, 499. |
| [162] | Meng Y.Y.; Wang M.; Jiang X.F. Angew. Chem., Int. Ed. 2020, 59, 1346. |
| [163] | Cui H.H.; Wei W.; Yang D.S.; Zhang Y.L.; Zhao H.J.; Wang L.L.; Wang H. Green Chem. 2017, 19, 3520. |
| [164] | Wang J.L.; Huang B.B.; Yang C.; Xia W.J. Chem. Commun. 2019, 55, 11103. |
| [165] | Wu J.; Zhang Y.L.; Gong X.C.; Meng Y.G.; Zhu C.Y. Org. Biomol. Chem. 2019, 17, 3507. |
| [166] | Gallhof M.; Kell L.; Brasholz M. Chem.-Eur. J. 2020, 26, 1772. |
| [167] | Wang Y.; Deng L.L.; Mei H.B.; Du B.N.; Han J.L.; Pan Y. Green Chem. 2018, 20, 3444. |
| [168] | Li D.D.; Li S.B.; Peng C.; Lu L.J.; Wang S.C.; Wang P.; Chen Y.-H.; Cong H.J.; Lei A.W. Chem. Sci. 2019, 10, 2791. |
| [169] | Luo M.-J.; Liu B.; Li Y.; Hu M.; Li J.-H. Adv. Synth. Catal. 2019, 361, 1538. |
| [170] | Zhu H.-D.; Hao W.-J.; Zhu C. - F.; Guo C.; Tu S.-J.; Jiang B. Org. Lett. 2020, 22, 4471. |
| [171] | Tang N.N.; Shao X.; Wang M.Y.; Wu X.X.; Zhu C. Acta Chim. Sinica 2019, 77, 922. (in Chinese) |
| [171] | ( 汤娜娜, 邵鑫, 王明扬, 吴新鑫, 朱晨, 化学学报, 2019, 77, 922.). |
| [172] | Yang J.H.; Fu X.B.; Lu Z.H.; Zhu G.G. Acta Chim. Sinica 2019, 77, 901. (in Chinese) |
| [172] | ( 杨俊航, 傅晓波, 卢增辉, 朱钢国, 化学学报, 2019, 77, 901.). |
/
| 〈 |
|
〉 |