有机化学 ›› 2021, Vol. 41 ›› Issue (1): 171-184.DOI: 10.6023/cjoc202006064 上一篇 下一篇
所属专题: 有机电合成虚拟专辑
综述与进展
王柏文a,b, 周永军a, 罗时荷a,b,*( ), 罗晓燕a, 陈伟清a, 杨诗敏a, 汪朝阳a,b,*(
), 罗晓燕a, 陈伟清a, 杨诗敏a, 汪朝阳a,b,*( )
)
收稿日期:2020-06-29
修回日期:2020-07-24
发布日期:2020-08-11
通讯作者:
罗时荷, 汪朝阳
作者简介:基金资助:
Bowen Wanga,b, Yongjun Zhoua, Shihe Luoa,b,*( ), Xiaoyan Luoa, Weiqing Chena, Shimin Yanga, Zhaoyang Wanga,b,*(
), Xiaoyan Luoa, Weiqing Chena, Shimin Yanga, Zhaoyang Wanga,b,*( )
)
Received:2020-06-29
Revised:2020-07-24
Published:2020-08-11
Contact:
Shihe Luo, Zhaoyang Wang
Supported by:文章分享
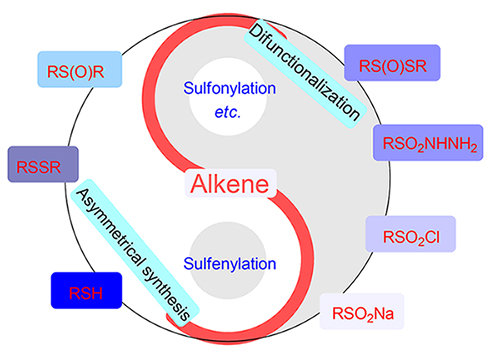
烯烃是重要的合成子, 其简洁高效的合成转化一直是有机合成领域的研究热点. 近年来, 基于烯烃骨架的C—S键成键反应特别受科学家的关注. 烯烃可以与常见的有机含硫试剂(包括硫醇或硫酚、二硫醚、亚砜、磺酰肼、磺酰氯和亚磺酸钠等)反应, 在烯烃的 α-位或者 β-位构筑C—S键来合成硫醚、亚砜或砜类化合物. 鉴于此, 以有机硫试剂的种类为分类依据, 综述了近年来非光催化与电化学体系下有机硫试剂参与的烯烃构建C—S反应的相关研究. 展望未来, 在烯烃与有机硫试剂的C—S成键反应研究中, 双官能团化反应和不对称催化合成具有较好的应用前景.
王柏文, 周永军, 罗时荷, 罗晓燕, 陈伟清, 杨诗敏, 汪朝阳. 非光催化与电化学体系下有机硫试剂参与的烯烃构建C—S键研究进展[J]. 有机化学, 2021, 41(1): 171-184.
Bowen Wang, Yongjun Zhou, Shihe Luo, Xiaoyan Luo, Weiqing Chen, Shimin Yang, Zhaoyang Wang. Research Progress in C—S Bond Formation Reaction of Olefins with Organic Sulfur Reagents under Photocatalyst-Free and Non-Electrochemical Conditions[J]. Chinese Journal of Organic Chemistry, 2021, 41(1): 171-184.
| [1] |
Trost B.M.; Kalnmals C.A. Chem.-Eur. J. 2019, 25, 11193.
|
| [2] |
Hofman K.; Liu N.-W.; Manolikakes G. Chem.-Eur. J. 2018, 24, 11852.
|
| [3] |
Mampuys P.; McElroy C.R.; Clark J.H.; Orru R.V.A.; Maes B.U.W. Adv. Synth. Catal. 2020, 362, 3.
|
| [4] |
Zhang S.N.; Yang S.H.; Huang L.H.; Zhao B.L.; Cheng K.; Qi C.Z. Chin. J. Org. Chem. 2015, 35, 2259. (in Chinese)
|
|
( 张诗浓, 杨胜虎, 黄乐浩, 赵保丽, 程凯, 齐陈泽, 有机化学, 2015, 35, 2259.).
|
|
| [5] |
Fang, Y.Y; Luo, Z.G.; Xu, X.M. RSC Adv. 2016, 6, 59661.
|
| [6] |
Huang G.B.; Li X.Y.; Luo J.R.; Luo Z.H.; Tan M.X. Chin. J. Org. Chem. 2019, 39, 617. (in Chinese)
|
|
( 黄国保, 李秀英, 罗金荣, 罗志辉, 谭明雄, 有机化学, 2019, 39, 617.).
|
|
| [7] |
Guo W.; Tao K.L.; Tan W.; Zhao M.M.; Zheng L.Y.; Fan X.L. Org. Chem. Front. 2019, 6, 2048.
|
| [8] |
Li Y.Q.; Fan Y.H. Synth. Commun. 2019, 49, 3227.
|
| [9] |
Cabrero-Antonino J.R.; Leyva-Perez A.; Corma A. Adv. Synth. Catal. 2012, 354, 678.
|
| [10] |
Mosaferi E.; Ripsman D.; Stephan D.W. Chem. Commun. 2016, 52, 8291.
|
| [11] |
Kucinski K.; Pawluc P.; Hreczycho G. Adv. Synth. Catal. 2015, 357, 3936.
|
| [12] |
Taniguchi N. ChemistrySelect 2018, 3, 6209.
|
| [13] |
Taniguchi N.; Kitayama K. Synlett 2018, 29, 2712.
|
| [14] |
Chun S.; Chung J.; Park J.E.; Chung Y.K. ChemCatChem 2016, 8, 2476.
|
| [15] |
Rosa C.H.; Peixoto M.L.B.; Rosa G.R.; Godoi B.; Galetto F.Z.; D'Oca M.G.M.; Godoi M. Tetrahedron Lett. 2017, 58, 3777.
|
| [16] |
Biermann U.; Metzger J.O. Eur. J. Org. Chem. 2018, 730.
|
| [17] |
Choudhuri K.; Mandal A.; Mal P. Chem. Commun. 2018, 54, 3759.
|
| [18] |
Lu Q.Q.; Wang H.M.; Peng P.; Liu C.; Huang Z.Y.; Luo Y.; Lei A.W. Org. Chem. Front. 2015, 2, 908.
|
| [19] |
Wang H.M.; Wang G.Y.; Lu Q.Q.; Chiang C.-W.; Peng P.; Zhou J.F.; Lei A.W. Chem.-Eur. J. 2016, 22, 14489.
|
| [20] |
Li C.S.; Li J.X.; Tan C.W.; Wu W.Q.; Jiang H.F. Org. Chem. Front. 2018, 5, 3158.
|
| [21] |
Zheng Y.; He Y.; Rong G.W.; Zhang X.L.; Weng Y.C.; Dong K.Y.; Xu X.F.; Mao J.C. Org. Lett. 2015, 17, 5444.
|
| [22] |
Cui H.H.; Liu X.X.; Wei W.; Yang D.S.; He C.L.; Zhang T.T.; Wang H. J. Org. Chem. 2016, 81, 2252.
|
| [23] |
Wang D.Y.; Yan Z.H.; Xie Q.H.; Zhang R.X.; Lin S.; Wang Y.X. Org. Biomol. Chem. 2017, 15, 1998.
|
| [24] |
Liu S.S.; Klussmann M. Chem. Commun. 2020, 56, 1557.
|
| [25] |
Zhou S.-F.; Pan X.Q.; Zhou Z.-H.; Shoberu A.; Zou J.-P. J. Org. Chem. 2015, 80, 3682.
|
| [26] |
Du B.N.; Wang Y.; Mei H.B.; Han J.L.; Pan Y. Adv. Synth. Catal. 2017, 359, 1684.
|
| [27] |
Zhao J.-J.; Tang M.; Zhang H.-H.; Xu M.-M.; Shi F. Chem. Commun. 2016, 52, 5953.
|
| [28] |
Hui Y.H.; Jiang J.; Wang W.T.; Chen W.L.; Cai Y.F.; Lin L.L.; Liu X.H.; Feng X.M. Angew. Chem., Int. Ed. 2010, 49, 4290.
|
| [29] |
Tian X.; Cassani C.; Liu Y.K.; Moran A.; Urakawa A.; Galzerano P.; Arceo E.; Melchiorre P. J. Am. Chem. Soc. 2011, 133, 17934.
|
| [30] |
Kitanosono T.; Sakai M.; Ueno M.; Kobayashi S. Org. Biomol. Chem. 2012, 10, 7134.
|
| [31] |
White J.D.; Shaw S. Chem. Sci. 2014, 5, 2200.
|
| [32] |
Chen J.A.; Meng S.X.; Wang L.M.; Tang H.M.; Huang Y. Chem. Sci. 2015, 6, 4184.
|
| [33] |
Farley A.J.M.; Sandford C.; Dixon D.J. J. Am. Chem. Soc. 2015, 137, 15992.
|
| [34] |
Huo C.D.; Wang Y.J.; Yuan Y.; Chen F.J.; Tang J. Chem. Commun. 2016, 52, 7233.
|
| [35] |
Xi H.; Deng B.C.; Zong Z.Z.; Lu S.L.; Li Z.P. Org. Lett. 2015, 17, 1180.
|
| [36] |
He L.; Guo H.; Li Y.-Z.; Du G.-F.; Dai B. Chem. Commun. 2014, 50, 3719.
|
| [37] |
Cong Z.-S.; Li Y.-G.; Du G.-F.; Gu C.-Z.; Dai B.; He L. Chem. Commun. 2017, 53, 13129.
|
| [38] |
Kawazoe S.; Yoshida K.; Shimazaki Y.; Oriyama T. Tetrahedron Lett. 2015, 56, 410.
|
| [39] |
Li Z.; Song G.Y.; He J.J.; Du Y.; Yang J.Y. J. Sulfur Chem. 2017, 38, 686.
|
| [40] |
Wei Q.; Hou W.D.; Liao N.; Peng Y.G. Adv. Synth. Catal. 2017, 359, 2364.
|
| [41] |
Wan J.-P.; Zhong S.S.; Xie L.L.; Cao X.J.; Liu Y.Y.; Wei L. Org. Lett. 2016, 18, 584.
|
| [42] |
Jiang Y.J.; Liang G.H.; Zhang C.; Loh T.-P. Eur. J. Org. Chem. 2016, 3326.
|
| [43] |
Siddaraju Y.; Prabhu K.R. J. Org. Chem. 2017, 82, 3084.
|
| [44] |
Orsi D.L.; Easley B.J.; Lick A.M.; Altman R.A. Org. Lett. 2017, 19, 1570.
|
| [45] |
Tamai T.; Ogawa A. J. Org. Chem. 2014, 79, 5028.
|
| [46] |
Tamai T.; Fujiwara K.; Higashimae S.; Nomoto A.; Ogawa A. Org. Lett. 2016, 18, 2114.
|
| [47] |
Yang X.-H.; Davison R.T.; Nie S.-Z.; Cruz F.A.; McGinnis T.M.; Dong V.M. J. Am. Chem. Soc. 2019, 141, 3006.
|
| [48] |
Yang X.-H.; Davison R.T.; Dong V.M. J. Am. Chem. Soc. 2018, 140, 10443.
|
| [49] |
Zhang Y.X.; Wong Z.R.; Wu X.X.; Lauw S.J.L.; Huang X.; Webster R.D.; Chi Y.G.R. Chem. Commun. 2016, 53, 184.
|
| [50] |
Wang L.L.; Yue H.L.; Yang D.S.; Cui H.H.; Zhu M.H.; Wang J.M.; Wei W.; Wang H. J. Org. Chem. 2017, 82, 6857.
|
| [51] |
Liang X.; Xiong M.T.; Zhu H.P.; Shen K.X.; Pan Y.J. J. Org. Chem. 2019, 84, 11210.
|
| [52] |
Wang Y.J.; Jiang W.; Huo C.D. J. Org. Chem. 2017, 82, 10628.
|
| [53] |
Singh A.K.; Chawla R.; Keshari T.; Yadav V.K.; Yadav L.D.S. Org. Biomol. Chem. 2014, 12, 8550.
|
| [54] |
Yang L.; Wen Q.; Xiao F.H.; Deng G.-J. Org. Biomol. Chem. 2014, 12, 9519.
|
| [55] |
Tu H.-Y.; Hu B.-L.; Deng C.-L.; Zhang X.-G. Chem. Commun. 2015, 51, 15558.
|
| [56] |
Liu Y.R.; Hu B.L.; Zhang X.G. Chin. J. Chem. 2017, 35, 307.
|
| [57] |
Sun K.; Lv Y.H.; Shi Z.D.; Mu S.Q.; Li C.H.; Wang X. Org. Biomol. Chem. 2017, 15, 5258.
|
| [58] |
Liu C.; Fang Y.; Wang S.-Y.; Ji S.-J. ACS Catal. 2019, 9, 8910.
|
| [59] |
Lin C.; Li D.Y.; Wang B.J.; Yao J.Z.; Zhang Y.H. Org. Lett. 2015, 17, 1328.
|
| [60] |
Liu S.-L.; Li X.-H.; Shi T.-H.; Yang G.-C.; Wang H.-L.; Gong J.-F.; Song M.-P. Eur. J. Org. Chem. 2017, 2280.
|
| [61] |
Shang Z.H.; Chen Q.Y.; Xing L.L.; Zhang Y.L.; Wait L.; Du Y.F. Adv. Synth. Catal. 2019, 361, 4926.
|
| [62] |
Liu C.; Fang Y.; Wang S.-Y.; Ji S.-J. Org. Lett. 2018, 20, 6112.
|
| [63] |
Tan Y.-H.; Li J.-X.; Hong W.-K.; Wang Z.-Y. Chin. J. Org. Chem. 2011, 31, 616. (in Chinese)
|
|
( 谭越河, 李建晓, 洪文坤, 汪朝阳, 有机化学, 2011, 31, 616.).
|
|
| [64] |
Mao C.-X.; Wang Z.-Y.; Tan Y.-H.; Xue F.-L. Chin. J. Org. Chem. 2011, 31, 1377. (in Chinese)
|
|
( 毛超旭, 汪朝阳, 谭越河, 薛福玲, 有机化学, 2011, 31, 1377.).
|
|
| [65] |
Liu J.; Chen W.; Wang L. RSC Adv. 2013, 3, 4723.
|
| [66] |
Cao L.; Luo S.-H.; Jiang K.; Hao Z.-F.; Wang B.-W.; Pang C.-M.; Wang Z.-Y. Org. Lett. 2018, 20, 4754.
|
| [67] |
Li J.; Rao W.D.; Wang S.-Y.; Ji S.-J. J. Org. Chem. 2019, 84, 11542.
|
| [68] |
Reddy R.J.; Kumar J.J.; Kumari A.H. Eur. J. Org. Chem. 2019, 3771.
|
| [69] |
Kong W.G.; Yu C.J.; An H.J.; Song Q.L. Org. Lett. 2018, 20, 4975.
|
| [70] |
Shyam P.K.; Son S.; Jang H.-Y. Eur. J. Org. Chem. 2017, 5025.
|
| [71] |
Son S.; Shyam P.K.; Park H.; Jeong I.; Jang H.-Y. Eur. J. Org. Chem. 2018, 3365.
|
| [72] |
Reddy R.J.; Kumari A.H.; Kumar J.J.; Nanubolu J.B. Adv. Synth. Catal. 2019, 361, 1587.
|
| [73] |
Shukla G.; Srivastava A.; Nagaraju A.; Raghuvanshi K.; Singh M.S. Adv. Synth. Catal. 2015, 357, 3969.
|
| [74] |
Wang B.-W.; Liu Y.; Hao Z.-F.; Hou J.-Q.; Li J.-Y.; Li S.-T.; Pan S.-H.; Zeng M.-H.; Wang Z.-Y. Chin. J. Org. Chem. 2018, 38, 1872. (in Chinese)
|
|
( 王柏文, 刘园, 郝志峰, 侯佳琦, 李健怡, 李舒婷, 潘思慧, 曾铭豪, 汪朝阳, 有机化学, 2018, 38, 1872.).
|
|
| [75] |
Yang K.; Gao J.-J.; Luo S.-H.; Wu H.-Q.; Pang C.-M.; Wang B.-W.; Chen X.-Y.; Wang Z.-Y. RSC Adv. 2019, 9, 19917.
|
| [76] |
Wu H.-Q.; Yang K.; Chen X.-Y.; Arulkumar M.; Wang N.; Chen S.-H.; Wang Z.-Y. Green Chem. 2019, 21, 3782.
|
| [77] |
Cao L.; Luo S.-H.; Wu H.-Q.; Chen L.-Q.; Jiang K.; Hao Z.-F.; Wang Z.-Y. Adv. Synth. Catal. 2017, 359, 2961.
|
| [78] |
Gao X.F.; Pan X.J.; Gao J.; Jiang H.F.; Yuan G.Q.; Li Y.W. Org. Lett. 2015, 17, 1038.
|
| [79] |
Hua J.W.; Xu J.Q.; Xu J.; Zhou B.C.; Zhang D.; Yang Z.; Fang Z.; Guo K. Eur. J. Org. Chem. 2019, 4056.
|
| [80] |
He R.F.; Chen X.W.; Li Y.B.; Liu Q.; Liao C.S.; Chen L.; Huang Y.B. J. Org. Chem. 2019, 84, 8750.
|
| [81] |
Ebule R.; Hammond G.B.; Xu B. Eur. J. Org. Chem. 2018, 4705.
|
| [82] |
Lan L.Y.; Gao Y.; Ding R.; Zhang T.; Liu Y.G.; Sun B.G.; Tian H.Y. Synth. Commun. 2019, 49, 539.
|
| [83] |
Gao X.F.; Pan X.J.; Gao J.; Huang H.W.; Yuan G.Q.; Li Y.W. Chem. Commun. 2015, 51, 210.
|
| [84] |
Jiang Y.J.; Loh T.-P. Chem. Sci. 2014, 5, 4939.
|
| [85] |
Shao A.L.; Gao M.; Chen S.T.; Wang T.; Lei A.W. Chem. Sci. 2017, 8, 2175.
|
| [86] |
Huang J.J.; Hu G.; An S.Y.; Chen D.D.; Li M.L.; Li P.F. J. Org. Chem. 2019, 84, 9758.
|
| [87] |
Yan J.J.; Pulis A.P.; Perry G.J.P.; Procter D.I. Angew. Chem., Int. Ed. 2019, 58, 15675.
|
| [88] |
Haut F.-L.; Habiger C.; Speck K.; Wurst K.; Mayer P.; Korber J.N.; Muller T.; Magauer T. J. Am. Chem. Soc. 2019, 141, 13352.
|
| [89] |
Xu X.M.; Li J.Z.; Wang Z.L. Chin. J. Org. Chem. 2020, 40, 886. (in Chinese)
|
|
( 徐鑫明, 李家柱, 王祖利, 有机化学, 2020, 40, 886.).
|
|
| [90] |
Deng L.L.; Liu Y.Y. ACS Omega 2018, 3, 11890.
|
| [91] |
Wang F.-X.; Tian S.-K. J. Org. Chem. 2015, 80, 12697.
|
| [92] |
Yang F.-L.; Wang F.-X.; Wang T.-T.; Wang Y.-J.; Tian S.-K. Chem. Commun. 2014, 50, 2111.
|
| [93] |
Yang F.-L.; Gui Y.; Yu B.-K.; Jin Y.-X.; Tian S.-K. Adv. Synth. Catal. 2016, 358, 3368.
|
| [94] |
Singh R.; Allam B.K.; Singh N.; Kumari K.; Singh S.K.; Singh K.N. Org. Lett. 2015, 17, 2656.
|
| [95] |
Aegurla B.; Peddinti R.K. Asian J. Org. Chem. 2018, 7, 946.
|
| [96] |
Liu C.R.; Ding L.H.; Guo G.; Liu W.W. Eur. J. Org. Chem. 2016, 910.
|
| [97] |
Wei W.; Liu C.L.; Yang D.S.; Wen J.W.; You J.M.; Suo Y.R.; Wang H. Chem. Commun. 2013, 49, 10239.
|
| [98] |
Wan X.; Sun K.; Zhang G.S. Sci. China: Chem. 2017, 60, 353.
|
| [99] |
Tang Y.C.; Fan Y.Y.; Gao H.J.; Li X.Q.; Xu X.S. Tetrahedron Lett. 2015, 56, 5616.
|
| [100] |
Tang Y.C.; Zhang Y.; Wang K.F.; Li X.Q.; Xu X.S.; Du X.H. Org. Biomol. Chem. 2015, 13, 7084.
|
| [101] |
Chen W.T.; Liu X.Y.; Chen E.; Chen B.H.; Shao J.A.; Yu Y.P. Org. Chem. Front. 2017, 4, 1162.
|
| [102] |
Terent'ev A.O.; Mulina O.M.; Pirgach D.A.; Demchuk D.V.; Syroeshkin M.A.; Nikishin G.I. RSC Adv. 2016, 6, 93476.
|
| [103] |
Choudhuri K.; Achar T.K.; Mal P. Adv. Synth. Catal. 2017, 359, 3566.
|
| [104] |
Sun K.; Lv Y.H.; Zhu Z.H.; Jiang Y.Q.; Qi J.J.; Wu H.K.; Zhang Z.G.; Zhang G.S.; Wang X. RSC Adv. 2015, 5, 50701.
|
| [105] |
Xu R.; Li Z.P. Tetrahedron Lett. 2018, 59, 3942.
|
| [106] |
Wang B.; Tang L.; Liu L.Y.; Li Y.N.; Yang Y.; Wang Z.Y. Green Chem. 2017, 19, 5794.
|
| [107] |
Wang D.Y.; Zhang R.X.; Lin S.; Yan Z.H.; Guo S.M. Synlett 2016, 27, 2003.
|
| [108] |
Ni J.X.; Jiang Y.; An Z.Y.; Lan J.F.; Yan R.L. Chem. Commun. 2019, 55, 7343.
|
| [109] |
Jiang L.Q.; Ding T.Q.; Yi W.-B.; Zeng X.; Zhang W. Org. Lett. 2018, 20, 2236.
|
| [110] |
Wei J.J.; Liang S.S.; Jiang L.Q.; Mumtaz Y.; Yi W.-B. J. Org. Chem. 2020, 85, 977.
|
| [111] |
Xia Y.Y.; Chen X.L.; Qu L.B.; Sun K.; Xia X.Y.; Fu W.K.; Chen X.; Yang Y.K.; Zhao Y.F.; Li C.J. Asian J. Org. Chem. 2016, 5, 878.
|
| [112] |
Lai M.; Wu Z.Y.; Li S.-J.; Wei D.H.; Zhao M.Q. J. Org. Chem. 2019, 84, 11135.
|
| [113] |
Zhu R.; Buchwald S.L. J. Am. Chem. Soc. 2015, 137, 8069.
|
| [114] |
Shi J.; Tang X.-D.; Wu Y.-C.; Li H.-N.; Song L.-J.; Wang Z.-Y. Eur. J. Org. Chem. 2015, 1193.
|
| [115] |
Lin Y.-M.; Lu G.-P.; Wang G.-X.; Yi W.-B. J. Org. Chem. 2017, 82, 382.
|
| [116] |
Wang D.Y.; Zhang R.X.; Ning W.; Yan Z.H.; Lin S. Org. Biomol. Chem. 2016, 14, 5136.
|
| [117] |
Wang B.-W.; Jiang K.; Li J.-X.; Luo S.-H.; Wang Z.-Y.; Jiang H.-F. Angew. Chem., Int. Ed. 2020, 59, 2338.
|
| [118] |
Shi J.; Tang X.-D.L; Wu Y.-C.; Fang J.-F.; Cao L.; Chen X.-Y.; Wang Z.-Y. RSC Adv. 2016, 6, 25651.
|
| [119] |
Zhang N.; Yang D.S.; Wei W.; Yuan L.; Cao Y.J.; Wang H. RSC Adv. 2015, 5, 37013.
|
| [120] |
Hua L.-N.; Li H.; Qing F.-L.; Huang Y.G.; Xu X.-H. Org. Biomol. Chem. 2016, 14, 8443.
|
| [121] |
Wu H.-Q.; Luo S.-H.; Cao L.; Shi H.-N.; Wang B.-W.; Wang Z.-Y. Asian J. Org. Chem. 2018, 7, 2479.
|
| [122] |
Wu H.-Q.; Yang K.; Luo S.-H.; Wu X.-Y.; Wang N.; Chen S.-H.; Wang Z.-Y. Eur. J. Org. Chem. 2019, 28, 4572.
|
| [123] |
Wu Y.-C.; Luo S.-H.; Mei W.-J.; Cao L.; Wu H.-Q.; Wang Z.-Y. Eur. J. Med. Chem. 2017, 139, 84.
|
| [124] |
Wu Y.-C.; Cao L.; Mei W.-J.; Wu H.-Q.; Luo S.-H.; Zhan H.-Y.; Wang Z.-Y. Chem. Biol. Drug Des. 2018, 92, 1232.
|
| [125] |
Luo S.-H.; Yang K.; Lin J.-Y.; Gao J.-J.; Wu X.-Y.; Wang Z.-Y. Org. Biomol. Chem. 2019, 17, 5138.
|
| [126] |
Cao L.; Li J.-X.; Wu H.-Q.; Jiang K.; Hao Z.-F.; Luo S.-H.; Wang Z.-Y. ACS Sustainable Chem. Eng. 2018, 6, 4147.
|
| [127] |
Ke M.L.; Huang G.X.; Ding L.; Fang J.J.; Chen F.-E. ChemCatChem 2019, 11, 4720.
|
| [128] |
Wang L.-J.; Chen M.M.; Qi L.; Xu Z.D.; Li W. Chem. Commun. 2017, 53, 2056.
|
| [129] |
Kariya A.; Yamaguchi T.; Nobuta T.; Tada N.; Miura T.; Itoh A. RSC Adv. 2014, 4, 13191.
|
| [130] |
Chawla R.; Singh A.K.; Yadav L.D.S. Eur. J. Org. Chem. 2014, 2032.
|
| [131] |
Zeng K.; Chen L.; Chen Y.; Liu Y.P.; Zhou Y.B.; Au C.-T.; Yin S.-F. Adv. Synth. Catal. 2017, 359, 841.
|
| [132] |
Xiong Y.; Sun Y.W.; Zhang G.Z. Org. Lett. 2018, 20, 6250.
|
| [133] |
Kramer P.; Halaczkiewicz M.; Sun Y.; Kelm H.; Manolikakes G. J. Org. Chem. 2020, 85, 3617.
|
| [134] |
Gao W.-C.; Cheng Y.-F.; Shang Y.-Z.; Chang H.-H.; Li X.; Zhou R.; Qiao Y.; Wei W.-L. J. Org. Chem. 2018, 83, 11956.
|
| [135] |
Shen T.; Yuan Y.Z.; Song S.; Jiao N. Chem. Commun. 2014, 50, 4115.
|
| [136] |
Wei W.; Wen J.W.; Yang D.S.; Du J.; You J.M.; Wang H. Green Chem. 2014, 16, 2988.
|
| [137] |
Chu X.-Q.; Meng H.; Xu X.-P.; Ji S.-J. Chem.-Eur. J. 2015, 21, 11359.
|
| [138] |
Wei W.; Wen J.W.; Yang D.S.; Wu M.; You J.M.; Wang H. Org. Biomol. Chem. 2014, 12, 7678.
|
| [139] |
Lu Q.Q.; Zhang J.; Wei F.L.; Qi Y.; Wang H.M.; Liu Z.L.; Lei A.W. Angew. Chem., Int. Ed. 2013, 52, 7156.
|
| [140] |
Yuan Z.L.; Wang H.-Y.; Mu X.; Chen P.H.; Guo Y.-L.; Liu G.S. J. Am. Chem. Soc. 2015, 137, 2468.
|
| [141] |
Liu X.; An R.; Zhang X.L.; Luo J.; Zhao X.D. Angew. Chem., Int. Ed. 2016, 55, 5846.
|
| [142] |
Luo J.; Liu Y.N.; Zhao X.D. Org. Lett. 2017, 19, 3434.
|
| [143] |
Zhang J.J.; Yang J.-D.; Zheng H.-L.; Xue X.-S.; Mayr H.; Cheng J.-P. Angew. Chem., Int. Ed. 2018, 57, 12690.
|
| [144] |
Luo J.; Cao Q.X.; Cao X.H.; Zhao X.D. Nat. Commun. 2018, 9, 527.
|
| [145] |
Liu X.; Lang Y.Y.; Ji J.Y.; Luo J.; Zhao X. J. Am. Chem. Soc. 2018, 140, 4782.
|
| [146] |
Zhang P.P.; Li M.; Xue X.-S.; Xu C.-F.; Zhao Q.C.; Liu Y.F.; Wang H.Y.; Guo Y.L.; Lu L.; Shen Q.L. J. Org. Chem. 2016, 81, 7486.
|
| [147] |
Yoo J.; Ha H.-J.; Kim B.; Cho C.-W. J. Org. Chem. 2020, 85, 7077.
|
| [148] |
Tian H.; Yu J.P.; Yang H.J.; Zhu C.J.; Fu H. Adv. Synth. Catal. 2016, 358, 1794.
|
| [149] |
Xiao Q.; He Q.J.; Li J.C.; Wang J. Org. Lett. 2015, 17, 6090.
|
| [150] |
Jia Y.M.; Qin H.M.; Wang N.; Jiang Z.-X.; Yang Z.G. J. Org. Chem. 2018, 83, 2808.
|
| [151] |
Sicignano M.; Rodriguez R.I.; Capaccio V.; Borello F.; Cano R.; De Riccardis F.; Bernardi L.; Diaz-Tendero S.; Della Sala G.; Aleman J. Org. Biomol. Chem. 2020, 18, 2914.
|
| [152] |
Jin M.Y.; Li J.C.; Huang R.K.; Zhou Y.X.; Chung L.W.; Wang J. Chem. Commun. 2018, 54, 4581.
|
| [153] |
Liang Y.Y.; Zhao X.D. ACS Catal. 2019, 9, 6896.
|
| [154] |
Xu C.F.; Shen Q.L. Org. Lett. 2015, 17, 4561.
|
| [155] |
Yang T.; Lu L.; Shen Q.L. Chem. Commun. 2015, 51, 5479.
|
| [156] |
Luo J.; Zhu Z.C.; Liu Y.N.; Zhao X.D. Org. Lett. 2015, 17, 3620.
|
| [157] |
Zhu Z.C.; Luo J.; Zhao X.D. Org. Lett. 2017, 19, 4940.
|
| [158] |
Song T.; Zhao X.M.; Wang X.L. J. Org. Chem. 2019, 84, 15648.
|
| [159] |
Nguyen T.B. Adv. Synth. Catal. 2020, 362, 3448.
|
| [160] |
Yin F.; Wang X.-S. Org. Lett. 2014, 16, 1128.
|
| [161] |
Cheng Z.-F.; Tao T.-T.; Feng Y.-S.; Tang W.-K.; Xu J.; Dai J.-J.; Xu H.-J. J. Org. Chem. 2018, 83, 499.
|
| [162] |
Meng Y.Y.; Wang M.; Jiang X.F. Angew. Chem., Int. Ed. 2020, 59, 1346.
|
| [163] |
Cui H.H.; Wei W.; Yang D.S.; Zhang Y.L.; Zhao H.J.; Wang L.L.; Wang H. Green Chem. 2017, 19, 3520.
|
| [164] |
Wang J.L.; Huang B.B.; Yang C.; Xia W.J. Chem. Commun. 2019, 55, 11103.
|
| [165] |
Wu J.; Zhang Y.L.; Gong X.C.; Meng Y.G.; Zhu C.Y. Org. Biomol. Chem. 2019, 17, 3507.
|
| [166] |
Gallhof M.; Kell L.; Brasholz M. Chem.-Eur. J. 2020, 26, 1772.
|
| [167] |
Wang Y.; Deng L.L.; Mei H.B.; Du B.N.; Han J.L.; Pan Y. Green Chem. 2018, 20, 3444.
|
| [168] |
Li D.D.; Li S.B.; Peng C.; Lu L.J.; Wang S.C.; Wang P.; Chen Y.-H.; Cong H.J.; Lei A.W. Chem. Sci. 2019, 10, 2791.
|
| [169] |
Luo M.-J.; Liu B.; Li Y.; Hu M.; Li J.-H. Adv. Synth. Catal. 2019, 361, 1538.
|
| [170] |
Zhu H.-D.; Hao W.-J.; Zhu C. - F.; Guo C.; Tu S.-J.; Jiang B. Org. Lett. 2020, 22, 4471.
|
| [171] |
Tang N.N.; Shao X.; Wang M.Y.; Wu X.X.; Zhu C. Acta Chim. Sinica 2019, 77, 922. (in Chinese)
|
|
( 汤娜娜, 邵鑫, 王明扬, 吴新鑫, 朱晨, 化学学报, 2019, 77, 922.).
|
|
| [172] |
Yang J.H.; Fu X.B.; Lu Z.H.; Zhu G.G. Acta Chim. Sinica 2019, 77, 901. (in Chinese)
|
|
( 杨俊航, 傅晓波, 卢增辉, 朱钢国, 化学学报, 2019, 77, 901.).
|
| [1] | 李思达, 崔鑫, 舒兴中, 吴立朋. 钛催化的烯烃制备1,1-二硼化合物[J]. 有机化学, 2024, 44(2): 631-637. |
| [2] | 陈雯雯, 张琴, 张松月, 黄芳芳, 张馨尹, 贾建峰. 无光催化剂条件下可见光诱导炔基碘和亚磺酸钠偶联反应[J]. 有机化学, 2024, 44(2): 584-592. |
| [3] | 张建涛, 张聪, 莫诺琳, 罗佳婷, 陈莲芬, 刘卫兵. 氯仿参与的烯烃自由基加成反应的研究进展[J]. 有机化学, 2023, 43(9): 3098-3106. |
| [4] | 石义军, 孙馨悦, 曹晗, 别福升, 马杰, 刘哲, 丛兴顺. 室温下酯与伯硫醇的硫酯化反应[J]. 有机化学, 2023, 43(7): 2499-2505. |
| [5] | 陆晓雨, 孙晓梅, 钮亚琴, 王俊超, 殷文婧, 高梦婷, 刘孜, 韦正桓, 陶庭骅. 铜催化氟代丙烯酸与氧杂吖丙啶的脱羧交叉偶联反应[J]. 有机化学, 2023, 43(6): 2110-2119. |
| [6] | 卢凯, 屈浩琦, 陈樨, 秋慧, 郑晶, 马猛涛. 无催化剂、无溶剂条件下炔烃和烯烃与儿茶酚硼烷的硼氢化反应[J]. 有机化学, 2023, 43(6): 2197-2205. |
| [7] | 刘甜甜, 张鸿鹏, 焦晓梦, 白银娟. 多信号同时检测生物硫醇荧光探针的研究进展[J]. 有机化学, 2023, 43(6): 2081-2095. |
| [8] | 李思达, 舒兴中, 吴立朋. 锆、钛介导的烯烃、炔烃硼氢化[J]. 有机化学, 2023, 43(5): 1751-1760. |
| [9] | 高师泉, 刘闯军, 杨俊锋, 张俊良. 钴催化的烯烃和炔烃的电化学还原偶联反应[J]. 有机化学, 2023, 43(4): 1559-1565. |
| [10] | 梁志鹏, 叶浩, 张海滨, 姜国民, 吴新星. 环丁酮类腙参与的偕二氟环丙烷开环胺化反应[J]. 有机化学, 2023, 43(4): 1483-1491. |
| [11] | 张妍妍, 张珠珠, 朱圣卿, 储玲玲. 镍催化不对称酰基化反应研究进展[J]. 有机化学, 2023, 43(3): 1023-1035. |
| [12] | 李落墨, 杨小会. 离子转移反应的研究进展[J]. 有机化学, 2023, 43(3): 1036-1044. |
| [13] | 郭萍, 周勇, 赵杰. 多取代烯烃的Z∶E高选择性合成制备[J]. 有机化学, 2023, 43(3): 855-872. |
| [14] | 侯虹宇, 程元元, 陈彬, 佟振合, 吴骊珠. 光催化烯烃α-酰化反应[J]. 有机化学, 2023, 43(3): 1012-1022. |
| [15] | Yasir Mumtaz, 刘杰, 黄鑫. 铜促进的苯胺类化合物与CF3SO2Na的三氟甲硫基化反应[J]. 有机化学, 2023, 43(2): 679-685. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||