

脱烯丙基反应研究进展
收稿日期: 2020-09-13
修回日期: 2020-10-28
网络出版日期: 2020-11-19
基金资助
国家自然科学基金(21672143); 上海交通大学医工交叉(YG2017MS26)
Advances in Deallylation
Received date: 2020-09-13
Revised date: 2020-10-28
Online published: 2020-11-19
Supported by
National Natural Science Foundation of China(21672143); Interdisciplinary Program of Shanghai Jiao Tong University(YG2017MS26)
烯丙基是有机合成中常用的保护基团, 具有引入简单, 在酸/碱性及还原剂等条件下稳定, 在相对温和的条件下选择性地脱保护等特点, 在有机合成特别是药物和天然产物的合成研究中具有重要地位. 近几十年来, 研究者们对各类烯丙基的脱保护方法进行了广泛研究. 按碱及还原剂促进、氧化及自由基过程、路易斯酸促进、碘促进、过渡金属催化及电化学方法等分类, 对脱烯丙基保护方法的研究进展进行了综述.
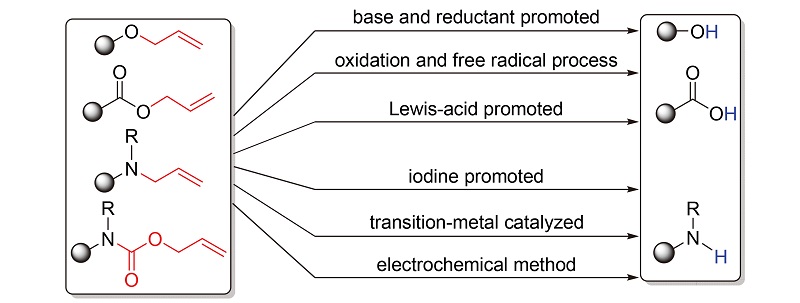
关键词: 脱烯丙基反应; 碱促进脱烯丙基化; 过渡金属催化脱烯丙基化; 氧化脱烯丙基化; 自由基历程脱烯丙基化; 路易斯酸催化脱烯丙基化; 电化学脱烯丙基化
王宇 , 王泾洋 , 吴啸宇 , 丁广妮 , 张兆国 , 谢小敏 . 脱烯丙基反应研究进展[J]. 有机化学, 2021 , 41(4) : 1337 -1358 . DOI: 10.6023/cjoc202009031
Allyl groups, as a kind of universal protective groups in organic synthesis, are easily introduced, and stable under acidic, basic and reductive conditions. Moreover, the deallylation may occur efficiently and selectively under mild conditions. Therefore, functional group protection with allyl moiety plays a significant role in organic synthesis, especially in the synthesis of natural products and pharmaceutical industry. In recent decades, various methods of deallylation have been developed. Herein, the comprehensive development on the deallylation reaction with base and reductant, oxidation and free radical, Lewis-acid, iodine, transition metals, and electrochemical methods is reviewed.

| [1] | Greene, T.W.; Wuts, P.G. M., Protective Groups in Organic Synthesis, 3rd ed., John Wiley & Sons, Inc., New York, 1999. |
| [2] | (a) Weissman, S.A.; Zewge, D. Tetrahedron 2005, 61,7833. |
| [2] | (b) Tang, J.-Y.; Liu, H.-X.; Huang, C.-S. Technol. Dev. Chem. Ind. 2016, 45,15. (in Chinese) |
| [2] | ( 唐剑耀, 刘红星, 黄初升, 化学技术与开发, 2016, 45,15.) |
| [2] | (c) Li, Z.J.; Zhang, S.Q.; Wang, A.B.; Cai, M.S. Acta Chim. Sinica 1998, 56,1128. (in Chinese) |
| [2] | ( 李中军, 张三奇, 王安邦, 蔡孟深, 化学学报, 1998, 56,1128.) |
| [2] | (d) Zhou, Y.; Zhang, L.R.; Zhang, L.H. Acta Chim. Sinica 2001, 59,1691. (in Chinese) |
| [2] | ( 周英, 张亮仁, 张礼和, 化学学报, 2001, 59,1691.) |
| [2] | (e) Deng, X.; Liu, W.; Li, C.; Zhang, Z.; Wang, X.; Liu, J. Chin. J. Org. Chem. 2011, 31,75. (in Chinese) |
| [2] | ( 邓喜玲, 刘卫东, 李超, 张志丽, 王孝伟, 刘俊义, 有机化学, 2011, 31,75.) |
| [3] | (a) Zhang, L.; Wang, Y.; Yu, J.; Zhang, G.; Cai, X.; Wu, Y.; Wang, L. Tetrahedron Lett. 2013, 54,4019. |
| [3] | (b) Takagi, K.; Fukuda, H.; Shuto, S.; Otaka, A.; Arisawa, M. Adv. Synth. Catal. 2015, 357,2119. |
| [3] | (c) Bu, X.; Williams, M.; Jo, J.; Koide, K.; Welch, C.J. Chem. Commun. 2017, 53,720. |
| [4] | Prosser, T.J. J. Am. Chem. Soc. 1961, 83,1701. |
| [5] | Price, C.C.; Snyder, W.H. J. Am. Chem. Soc. 1961, 83,1773. |
| [6] | (a) Oltvoort, J.J.; Kloosterman, M.; van Boom, J.H. Recl. Trav. Chim. Pays-Bas 1983, 102,501. |
| [6] | (b) Guibe, F.; M'Leux, Y.S. Tetrahedron Lett. 1981, 22,3591. |
| [7] | Gevorgyan, V.; Yamamoto, Y. Tetrahedron Lett. 1995, 36,7765. |
| [8] | Nicolaou, K.C.; Caulfield, T.J.; Kataoka, H.; Stylianides, N.A. J. Am. Chem. Soc. 1990, 112,3693. |
| [9] | Gigg, R.; Warren, C.D. J. Chem. Soc. C 1968,1903. |
| [10] | Halkes, K.M.; Slaghek, T.M.; Vermeer, H.J.; Kamerling, J.P.; Vliegenthart, J.F. G. Tetrahedron Lett. 1995, 36,6137. |
| [11] | Mereyala, H.B.; Lingannagaru, S.R. Tetrahedron 1997, 53,17501. |
| [12] | Cunningham, J.; Gigg, R.; Warren, C.D. Tetrahedron Lett. 1964, 5,1191. |
| [13] | (a) Gigg, J.; Gigg, R. J. Chem. Soc. C 1966,82. |
| [13] | (b) Smith, A.B.; Rivero, R.A.; Hale, K.J.; Vaccaro, H.A. J. Am. Chem. Soc. 1991, 113,2092. |
| [14] | Yamada, H.; Harada, T.; Takahashi, T. J. Am. Chem. Soc. 1994, 116,7919. |
| [15] | Lamberth, C.; Bednarski, M.D. Tetrahedron Lett. 1991, 32,7369. |
| [16] | Pirrung, F.O. H.; Rutjes, F. P., J.T.; Hiemstra, H.; Speckamp, W.N. Tetrahedron Lett. 1990, 31,5365. |
| [17] | Effenberger, F.; J?ger, J. J. Org. Chem. 1997, 62,3867. |
| [18] | Kametani, T.; Huang, S.-P.; Ihara, M.; Fukumoto, K. J. Org. Chem. 1976, 41,2545. |
| [19] | Thomas, R.M.; Mohan, G.H.; Iyengar, D.S. Tetrahedron Lett. 1997, 38,4721. |
| [20] | Li, C.B.; Ji, X.J.; Zhang, S.M.; Lu, M.; Zhao, Z.X.; Cui, Y.; Xu, Y.L.; Yang, Q.C.; Zhang, W.Q. Chin. Chem. Lett. 2003, 14,459. |
| [21] | Pawar, B.V.; Lokhande, P.D. Synth. Commun. 2009, 39,2445. |
| [22] | Mann, F.G.; Pragnell, M.J. J. Chem. Soc. 1965,4120. |
| [23] | Bailey, W.F.; England, M.D.; Mealy, M.J.; Thongsornkleeb, C.; Teng, L. Org. Lett. 2000, 2,489. |
| [24] | Sanz, R.; Martinez, A.; Marcos, C.; Fananas, F.J. Synlett 2008,1957. |
| [25] | Alonso, E.; Ramón, D.J.; Yus, M. Tetrahedron 1997, 53,14355. |
| [26] | Kariyone, K.; Yazawa, H. Tetrahedron Lett. 1970, 11,2885. |
| [27] | Choudary, B.M.; Prasad, A.D.; Swapna, V.; Valli, V.L. K.; Bhuma, V. Tetrahedron 1992, 48,953. |
| [28] | Yadav, J.S.; Chandrasekhar, S.; Sumithra, G.; Kache, R. Tetrahedron Lett. 1996, 37,6603. |
| [29] | Kumar, P.; Cherian, S.K.; Jain, R.; Show, K. Tetrahedron Lett. 2014, 55,7172. |
| [30] | Robles, Diaz, R.; Rodriguez Melgarejo, C.; Plaza Lopez-Espinosa, M.T.; Izquierdo Cubero, I. J. Org. Chem. 1994, 59,7928. |
| [31] | Yang, S.G.; Park, M.Y.; Kim, Y.H. Synlett 2002,492. |
| [32] | Dahlen, A.; Sundgren, A.; Lahmann, M.; Oscarson, S.; Hilmersson, G. Org. Lett. 2003, 5,4085. |
| [33] | Escoubet, S.; Gastaldi, S.; Timokhin, V.I.; Ber-trand, M.P.; Siri, D. J. Am. Chem. Soc. 2004, 126,12343. |
| [34] | Perchyonok, V.T.; Ryan, S.J.; Langford, S.J.; Hearn, M.T.; Tuck, K.L. Synlett 2008,1233. |
| [35] | Balgotra, S.; Venkateswarlu, V.; Vishwakarma, R.A.; Sawant, S.D. Tetrahedron Lett. 2015, 56,4289. |
| [36] | Garbers, C.F.; Steenkamp, J.A.; Visagie, H.E. Tetrahedron Lett. 1975, 16,3753. |
| [37] | Bhatt, M.V.; El-Morey, S.S. Synthesis 1982,1048. |
| [38] | Akiyama, T.; Hirofuji, H.; Ozaki, S. Tetrahedron Lett. 1991, 32,1321. |
| [39] | Sakate, S.S.; Kamble, S.B.; Chikate, R.C.; Rode, C.V. New J. Chem. 2017, 41,4943. |
| [40] | Nagaraju, M.; Krishnaiah, A.; Mereyala, H.B. Synth. Commun. 2007, 37,2467. |
| [41] | (a) Konda, S.G.; Humne, V.T.; Lokhande, P.D. Green Chem. 2011, 13,2354. |
| [41] | (b) Humne, V.T.; Hasanzadeh, K.; Lokhande, P.D. Res. Chem. Intermed. 2013, 39,585. |
| [41] | (c) Humne, V.; Lokahnde, P. Synth. Commun. 2014, 44,929. |
| [42] | Patil, A.M.; Kamble, D.A.; Lokhande, P.D. ChemistrySelect 2017, 2,8418. |
| [43] | Satyanarayana, K.; Chidambaram, N.; Chandra-sekaran, S. Synth. Commun. 1989, 19,2159. |
| [44] | Kadam, S.M.; Nayak, S.K.; Banerji, A. Tetrahedron Lett. 1992, 33,5129. |
| [45] | Talukdar, S.; Nayak, S.K.; Banerji, A. J. Org. Chem. 1998, 63,4925. |
| [46] | Lee, J.; Cha, J.K. Tetrahedron Lett. 1996, 37,3663. |
| [47] | Ohkubo, M.; Mochizuki, S.; Sano, T.; Kawaguchi, Y.; Okamoto, S. Org. Lett. 2007, 9,773. |
| [48] | Rajakumar, P.; Murali, V. Synth. Commun. 2003, 33,3891. |
| [49] | Ito, H.; Taguchi, T.; Hanzawa, Y. J. Org. Chem. 1993, 58,774. |
| [50] | Corey, E.J.; Suggs, J.W. J. Org. Chem. 1973, 38,3224. |
| [51] | (a) Gent, P.A.; Gigg, R. J. Chem. Soc., hem. Commun. 1974,277. |
| [51] | (b) Gigg, R. J. Chem. Soc., erkin Trans. 1 1980,738. |
| [52] | Sundberg, R.J.; Hamilton, G.S.; Laurino, J.P. J. Org. Chem. 1988, 53,976. |
| [53] | Ziegler, F.E.; Brown, E.G.; Sobolov, S.B. J. Org. Chem. 1990, 55,3691. |
| [54] | Zacuto, M.J.; Xu, F. J. Org. Chem. 2007, 72,6298. |
| [55] | Boss, R.; Scheffold, R. Angew. Chem., nt. Ed. 1976, 15,558. |
| [56] | Mori, M.; Ban, Y. Chem. Pharm. Bull. 1976, 24,1992. |
| [57] | Bieg, T.; Szeja, W. J. Carbohydr. Chem. 1985, 4,441. |
| [58] | Nakayama, K.; Uoto, K.; Higashi, K.; Soga, T.; Kusama, T. Chem. Pharm. Bull. 1992, 40,1718. |
| [59] | Mereyala, H.B.; Guntha, S. Tetrahedron Lett. 1993, 34,6929. |
| [60] | Honda, M.; Morita, H.; Nagakura, I. J. Org. Chem. 1997, 62,8932. |
| [61] | (a) Ishizaki, M.; Yamada, M.; Watanabe, S.-I.; Hoshino, O.; Nishitani, K.; Hayashida, M.; Tanaka, A.; Hara, H. Tetrahedron 2004, 60,7973. |
| [61] | (b) Yamada, M.; Watanabe, S.-I.; Hoshino, O.; Ishizaki, M.; Hayashida, M.; Tanaka, A.; Hara, H. Chem. Pharm. Bull. 2003, 51,1220. |
| [62] | (a) Hata, G.; Takahashi, K.; Miyake, A. J. Chem. Soc., hem. Commun. 1970,1392. |
| [62] | (b) Takahashi, K.; Miyake, A.; Hata, G. Bull. Chem. Soc. Jpn. 1972, 45,230. |
| [63] | Jeffrey, P.D.; McCombie, S.W. J. Org. Chem. 1982, 47,587. |
| [64] | Kunz, H.; Unverzagt, C. Angew. Chem., nt. Ed. 1984, 23,436. |
| [65] | Minami, I.; Ohashi, Y.; Shimizu, I.; Tsuji, J. Tetrahedron Lett. 1985, 26,2449. |
| [66] | (a) Four, P.; Guibe, F. Tetrahedron Lett. 1982, 23,1825. |
| [66] | (b) Dangles, O.; Guibé, F.; Balavoine, G.; Lavielle, S.; Marquet, A. J. Org. Chem. 1987, 52,4984. |
| [67] | Roos, E.C.; Bernabe, P.; Hiemstra, H.; Speckamp, W.N.; Kaptein, B.; Boesten, W.H. J. J. Org. Chem. 1995, 60,1733. |
| [68] | Deziel, R. Tetrahedron Lett. 1987, 28,4371. |
| [69] | Yamada, T.; Goto, K.; Mitsuda, Y.; Tsuji, J. Tetrahedron Lett. 1987, 28,4557. |
| [70] | Garro-Helion, F.; Merzouk, A.; Guibé, F. J. Org. Chem. 1993, 58,6109. |
| [71] | (a) Beugelmans, R.; Bourdet, S.; Bigot, A.; Zhu, J. Tetrahedron Lett. 1994, 35,4349. |
| [71] | (b) Beugelmans, R.; Neuville, L.; Bois-Choussy, M.; Chastanet, J.; Zhu, J. Tetrahedron Lett. 1995, 36,3129. |
| [72] | Lemaire-Audoire, S.; Savignac, M.; Genêt, J.P.; Bernard, J.-M. Tetrahedron Lett. 1995, 36,1267. |
| [73] | (a) Genêt, J.P.; Blart, E.; Savignac, M.; Lemeune, S.; Paris, J.-M. Tetrahedron Lett. 1993, 34,4189. |
| [73] | (b) Lemaire-Audoire, S.; Savignac, M.; Blart, E.; Pourcelot, G.; Genêt, J.P.; Bernard, J.-M. Tetrahedron Lett. 1994, 35,8783. |
| [73] | (c) Genêt, J.P.; Blart, E.; Savignac, M.; Lemeune, S.; Lemaire-Audoire, S.; Paris, J.-M.; Bernard, J.-M. Tetrahedron 1994, 50,497. |
| [73] | (d) Lemaire-Audoire, S.; Savignac, M.; Pourcelot, G.; Genet, J.-P.; Bernard, J.-M. J. Mol. Catal. A: Chem. 1997, 116,247. |
| [74] | Seki, M.; Kondo, K.; Kuroda, T.; Yamanaka, T.; Iwasaki, T. Synlett 1995,609. |
| [75] | Murakami, H.; Minami, T.; Ozawa, F. J. Org. Chem. 2004, 69,4482. |
| [76] | Mora, G.; Piechaczyk, O.; Le Goff, X.F.; Le Floch, P. Organometallics 2008, 27,2565. |
| [77] | Mao, Y.; Liu, Y.; Hu, Y.; Wang, L.; Zhang, S.; Wang, W. ACS Catal. 2018, 8,3016. |
| [78] | Enugala, R.; Carvalho, L.C. R.; Marques, M.M. B. Synlett 2010,2711. |
| [79] | Martínez-Calvo, M.; Couceiro, J.R.; Destito, P.; Rodríguez, J.; Mosquera, J.; Mascare?as, J.L. ACS Catal. 2018,8. |
| [80] | Garner, A.L.; Song, F.; Koide, K. J. Am. Chem. Soc. 2009, 131,5163. |
| [81] | Nieberding, M.; Tracey, M.P.; Koide, K. ACS Sens. 2017, 2,1737. |
| [82] | Jbara, M.; Eid, E.; Brik, A. Org. Biomol. Chem. 2018, 16,4061. |
| [83] | Alcaide, B.; Almendros, P.; Alonso, J.M. Chem.-Eur. J. 2003, 9,5793. |
| [84] | Alcaide, B.; Almendros, P.; Alonso, J.M. Chem.-Eur. J. 2006, 12,2874. |
| [85] | Tanaka, S.; Saburi, H.; Ishibashi, Y.; Kitamura, M. Org. Lett. 2004, 6,1873. |
| [86] | Tanaka, S.; Saburi, H.; Kitamura, M. Adv. Synth. Catal. 2006, 348,375. |
| [87] | Tanaka, S.; Saburi, H.; Murase, T.; Yoshimura, M.; Kitamura, M. J. Org. Chem. 2006, 71,4682. |
| [88] | Cadierno, V.; Garcia-Garrido, S.E.; Gimeno, J.; Nebra, N. Chem. Commun. 2005,4086. |
| [89] | Kamijo, S.; Huo, Z.; Jin, T.; Kanazawa, C.; Yamamoto, Y. J. Org. Chem. 2005, 70,6389. |
| [90] | Kajihara, K.; Arisawa, M.; Shuto, S. J. Org. Chem. 2008, 73,9494. |
| [91] | Sasmal, P.K.; Carregal-Romero, S.; Parak, W.J.; Meggers, E. Organometallics 2012, 31,5968. |
| [92] | Rodriguez, J.G.; Canoira, L. React. Kinet. Catal. Lett. 1989, 38,351. |
| [93] | Chouhan, M.; Kumar, K.; Sharma, R.; Grover, V.; Nair, V.A. Tetrahedron Lett. 2013, 54,4540. |
| [94] | Taniguchi, T.; Ogasawara, K. Angew. Chem., nt. Ed. 1998, 37,1136. |
| [95] | Taniguchi, T.; Ogasawara, K. Tetrahedron Lett. 1998, 39,4679. |
| [96] | Kim, S.; Jo, J.; Lee, D. Org. Lett. 2016, 18,4530. |
| [97] | Gaertner, D.; Konnerth, H.; von Wangelin, A.J. Catal. Sci. Technol. 2013, 3,2541. |
| [98] | Iqbal, J.; Srivastava, R.R. Tetrahedron 1991, 47,3155. |
| [99] | Giedyk, M.; Turkowska, J.; Lepak, S.; Mar-culewicz, M.; Proinsias, K.O.; Gryko, D. Org. Lett. 2017, 19,2670. |
| [100] | Hemming, D.S.; Talbot, E.P.; Steel, P.G. Tetrahedron Lett. 2017, 58,17. |
| [101] | Torii, S.; Tanaka, H.; Katoh, T.; Morisaki, K. Tetrahedron Lett. 1984, 25,3207. |
| [102] | Espanet, B.; Du?ach, E.; Périchon, J. Tetrahedron Lett. 1992, 33,2485. |
| [103] | Olivero, S.; Du?ach, E. J. Chem. Soc., hem. Commun. 1995,2497. |
| [104] | Yasuhara, A.; Kasano, A.; Sakamoto, T. J. Org. Chem. 1999, 64,4211. |
/
| 〈 |
|
〉 |