

利用芳基乙烷的脱氢硝化合成硝基芳香烯烃的新方法
收稿日期: 2020-08-24
修回日期: 2020-11-27
网络出版日期: 2021-02-07
基金资助
国家自然科学基金(21702191)
Alternative Approach for the Synthesis of Nitroaromatic Olefins via Dehydrogenative Nitration of Easily Available Arylethanes
Received date: 2020-08-24
Revised date: 2020-11-27
Online published: 2021-02-07
Supported by
the National Natural Science Foundation of China(21702191)
穆兵 , 吴俊良 , 张广安 . 利用芳基乙烷的脱氢硝化合成硝基芳香烯烃的新方法[J]. 有机化学, 2021 , 41(1) : 250 -257 . DOI: 10.6023/cjoc202008041
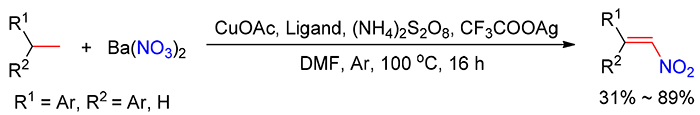
Nitroolefin is a common and versatile reagent, synthesis of which from aldehydes/ketones, α, β-unsaturated carboxylic acids or olefins is generally limited by the high cost of raw materials in industrial processes in the future. Herein, an alternative and economical protocol for the synthesis of nitroaromatic olefins directly from easily available arylethanes with barium nitrate using Cu/Ag as cocatalyst and ammonium persulfate as the terminal oxidant is reported. Additionally, 1,1-diphenylethanes, phenylethanes, 4-ethyl-1,1'-biphenyl and ethylnaphthalenes were suitable substrates for the current dehydrogenative nitration, and provided E-nitroaromatic olefins in moderate to good yields.
| [1] | Reddy M.A.; Jain N.; Yada D.; Kishore C.; Reddy V.J.; Reddy P.S.; Addlagatta A.; Kalivendi S.V.; Sreedhar B. J. Med. Chem. 2011, 54, 6751. |
| [2] | Lu L.Q.; Chen J.R.; Xiao W.J. Acc. Chem. Res. 2012, 45, 1278. |
| [3] | Kaap S.; Quentin I.; Tamiru D.; Shaheen M.; Eger K.; Steinfelder H.J. Biochem. Pharmacol. 2003, 65, 603. |
| [4] | Uehara H.; Imashiro R.; Hernández-Torres G.; Barbas III, C.F.Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 20672. |
| [5] | Meah Y.; Massey V. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 10733. |
| [6] | Ishii T.; Fujioka S.; Sekiguchi Y.; Kotsuki H. J. Am. Chem. Soc. 2004, 126, 9558. |
| [7] | Huang H.B.; Jacobsen E.N. J. Am. Chem. Soc. 2006, 128, 7170. |
| [8] | Tripathi C.B.; Kayal S.; Mukherjee S. Org. Lett. 2012, 14, 3296. |
| [9] | Bai B.; Wang L.; Yang J.; Cai L.L.; Liu Q.J.; Xi, Gao. L.; Zhao, Z.W.; Mao, D.B.; Chen, Z.F. Chin. J. Org. Chem. 2019, 39, 1053. (in Chinese) |
| [9] | ( 白冰, 王龙, 杨静, 蔡莉莉, 刘前进, 席高磊, 赵志伟, 毛多斌, 陈芝飞, 有机化学, 2019, 39, 1053.). |
| [10] | Luo S.P.; Wang L.P.; Yue H.D.; Le Z.G.; Yang W.L.; Xu D.Q.; Xu Z.Y. Acta Chim Sinica. 2006, 64, 1483. |
| [11] | Evans D.A.; Mito S.; Seidel D. J. Am. Chem. Soc. 2007, 129, 11583. |
| [12] | March J. Advanced Organic Chemistry, 3rd |
| [13] | Albrecht L.; Dickmeiss G.; Acosta F.C.; Rodríguez-Escrich C.; Davis R.L.; Jorgensen K.A. J. Am. Chem. Soc. 2012, 134, 2543. |
| [14] | Liu Y.K.; Nappi M.; Arceo E.; Vera S.; Melchiorre P. J. Am. Chem. Soc. 2011, 133, 15212. |
| [15] | Arai T.; Mishiro A.; Yokoyama N.; Suzuki K.; Sato H. J. Am. Chem. Soc. 2010, 132, 5338. |
| [16] | Denmark S.E.; Thorarensen A. Chem. Rev. 1996, 96, 137. |
| [17] | Yan L.J.; Xu H.; Wang Y.; Dong J.W.; Wang Y.C. Chin. J. Org. Chem. 2020, 40, 284. (in Chinese) |
| [17] | ( 严丽君, 徐菡, 王艳, 董建伟, 王永超, 有机化学, 2020, 40, 284.). |
| [18] | Basavaiah D.; Reddy B.S.; Badsara S.S. Chem. Rev. 2010, 110, 5447. |
| [19] | Nair D.K.; Mobin S.M.; Namboothiri I.N.N. Org. Lett. 2012, 14, 4580. |
| [20] | Quan X.J.; Ren Z.H.; Wang Y.Y.; Guan Z.H. Org. Lett. 2014, 16, 5728. |
| [21] | Chen Y.F.; Nie G.; Zhang Q.; Ma S.; Li H.; Hu Q.Q. Org. Lett. 2015, 17, 1118. |
| [22] | Kurti L.; Czako B. Strategic Applications of Named Reactions in Organic Synthesis, Elsevier Academic Press, London , 2005. |
| [23] | Fioravanti S.; Pellacani L.; Tardella P.A.; Vergari M.C. Org. Lett. 2008, 10, 1449. |
| [24] | Hassner A.; Kropp J.E.; Kent G.J. J. Org. Chem. 1969, 34, 2628. |
| [25] | Ranganathan S.; Kar S.K. J. Org. Chem. 1970, 35, 3962. |
| [26] | Corey E.J.; Estreicher H. J. Am. Chem. Soc. 1978, 100, 6294. |
| [27] | Barluenga J.; Rodríguez M.A.; Campos P.J.; Asensio G. J. Chem. Soc., Chem. Commun. 1987, 1491. |
| [28] | Barluenga J.; Rodríguez M.A.; Campos P.J. J. Chem. Soc., Perkin Trans. 1 1990, 2807. |
| [29] | Sy W.W.; By A.W. Tetrahedron Lett. 1985, 26, 1193. |
| [30] | Jew S.S.; Kim H.D.; Cho Y.S.; Cook C.H. Chem. Lett. 1986, 15, 1747. |
| [31] | Hwu J.R.; Chen K.L.; Ananthan S.; Patel H.V. Organometallics 1996, 15, 499. |
| [32] | Ghosh D.; Nichols D.E. Synthesis 1996, 195. |
| [33] | Suzuki H.; Mori T. J. Org. Chem. 1997, 62, 6498. |
| [34] | Mukaiyama T.; Hata E.; Yamada T. Chem. Lett. 1995, 24, 505. |
| [35] | Hata E.; Yamada T.; Mukaiyama T. Bull. Chem. Soc. Jpn. 1995, 68, 3629. |
| [36] | Jovel I.; Prateeptongkum S.; Jackstell R.; Vogl N.; Weckbecker C.; Beller M. Adv. Synth. Catal. 2008, 350, 2493. |
| [37] | Varma R.S.; Naicker K.P.; Liesent P.J. Tetrahedron Lett. 1998, 39, 3977. |
| [38] | Manna S.; Jana S.; Saboo T.; Maji A.; Maiti D. Chem. Commun. 2013, 49, 5286. |
| [39] | Rokade B.V.; Prabhu K.R. Org. Biomol. Chem. 2013, 11, 6713. |
| [40] | Das J.P.; Sinha P.; Roy S. Org. Lett. 2002, 4, 3055. |
| [41] | Baruah D.; Pahari P.; Konwar D. Tetrahedron Lett. 2015, 56, 2418. |
| [42] | Yang Z.; Li J.; Hua J.; Yang T.; Yi J.M.; Zhou C.S. Synlett 2017, 28, 1079. |
| [43] | Luo Z.G.; Xu F.; Fang Y.Y.; Liu P.; Xu X.M.; Feng C.T.; Li Z.; He J. Res. Chem. Intermed. 2016, 42, 6079. |
| [44] | Maity S.; Manna S.; Rana S.; Naveen T.; Mallick A.; Maiti D. J. Am. Chem. Soc. 2013, 135, 3355. |
| [45] | Naveen T.; Maity S.; Sharma U.; Maiti D. J. Org. Chem. 2013, 78, 5949. |
| [46] | Maity S.; Naveen T.; Sharma U.; Maiti D. Org. Lett. 2013, 15, 3384. |
| [47] | Zhao A.; Jiang Q.; Jia J.; Xu B.; Liu Y.F.; Zhang M.Z.; Liu Q.; Luo W.P.; Guo C.C. Tetrahedron Lett. 2016, 57, 80. |
| [48] | Whiting K.; Carmona L.G.; Sousa T. Renewable Sustainable Energy Rev. 2017, 76, 202. |
| [49] | Degnan T.F. J. Catal. 2003, 216, 32. |
| [50] | Grant J.T.; Venegas J.M.; McDermott W.P.; Hermans I. Chem. Rev. 2018, 118, 2769. |
| [51] | Kumar A.; Bhatti T.M.; Goldman A.S. Chem. Rev. 2017, 117, 12357. |
| [52] | Wang Y.L.; Qian L.; Huang Z.D.; Liu G.X.; Huang Z. Chin. J. Chem. 2020, 38, 837. |
| [53] | Sathyamoorthi S.; Banerjee S. ChemistrySelect 2017, 2, 10678. |
| [54] | Sathyamoorthi S.; Du Bois, J. Org. Lett. 2016, 18, 6308. |
| [55] | Banerjee S.; Sathyamoorthi S.; Du Bois J.; Zare R.N. Chem. Sci. 2017, 8, 7003. |
| [56] | Manna S.; Antonchick A.P. Chem. -Eur. J. 2017, 23, 7825. |
| [57] | Burkhard C.A.; Brown J.F., Jr. US 2867669, 1959. |
| [58] | Tang X.J.; Dolbier Jr.W.R. Angew. Chem. Int. Ed. 2015, 54, 4246. |
| [59] | Ambala S.; Singh R.; Singh M.; Cham P.S.; Gupta R.; Munagala G.; Yempalla K.R.; Vishwakarma R.A.; Singh P.P. RSC Adv. 2019, 9, 30428. |
| [60] | Huie R.E.; Clifton C.L.; Kafafi S.A. J. Phys. Chem. 1991, 95, 9336. |
| [61] | Huie R.E.; Clifton C.L. J. Phys. Chem. 1990, 94, 8561. |
| [62] | Liu Y.; Wang Q.L.; Chen Z.; Zhou Q.; Zhou C.S.; Xiong B.Q.; Zhang P.L.; Yang C.A.; Tang K.W. Org. Biomol. Chem. 2019, 17, 1365. |
| [63] | Manna S.; Jana S.; Saboo T.; Maji A.; Maiti D. Chem. Commun. 2013, 49, 5286. |
| [64] | Gross Z.; Hoz S. J. Am. Chem. Soc. 1988, 110, 7489. |
| [65] | Hsieh T.H.H.; Dong V.M. Tetrahedron 2009, 65, 3062. |
| [66] | Zhao A.; Jiang Q.; Jia J.; Xu, Bin.; Liu, Y.F.; Zhang, M.Z.; Liu, Q.; Luo, W.P.; Guo, C.C. Tetrahedron Lett. 2016, 57, 80. |
| [67] | Ambala S.; Singh R.; Singh M.; Cham P.S.; Gupta R.; Munagala G.; Yempalla K.R.; Vishwakarma R.A.; Singh P.P. RSC Adv. 2019, 9, 30428. |
/
| 〈 |
|
〉 |