有机化学 ›› 2021, Vol. 41 ›› Issue (1): 250-257.DOI: 10.6023/cjoc202008041 上一篇 下一篇
研究论文
收稿日期:2020-08-24
修回日期:2020-11-27
发布日期:2021-01-07
通讯作者:
吴俊良
作者简介:基金资助:
Bing Mua,b, Junliang Wua,*( ), Guang'an Zhanga
), Guang'an Zhanga
Received:2020-08-24
Revised:2020-11-27
Published:2021-01-07
Contact:
Junliang Wu
Supported by:文章分享
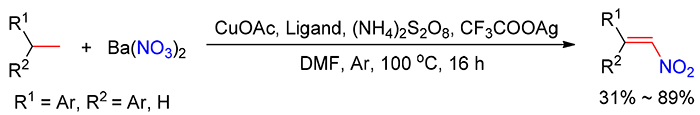
硝基烯烃是有机合成化学中常见的重要中间体, 其合成方法主要通过硝基烷烃与醛或酮的缩合、消除, 烯烃直接脱氢硝化或者烯基羧酸脱羧硝化得到目标产物, 但是这些合成方法由于原料价格昂贵, 在大规模生产中受到限制. 本研究首次采用廉价易得的芳基乙烷与硝酸钡为原料, 以铜/银为催化剂, 过硫酸钾为氧化剂, 通过脱氢硝化反应合成硝基芳香烯烃. 在优化的反应体系中, 1,1-二苯基乙烷、苯基乙烷、4-乙基联苯及乙基萘类化合物能与硝酸钡进行脱氢硝化反应, 以中等至好的收率获得 E型硝基芳香烯烃.
穆兵, 吴俊良, 张广安. 利用芳基乙烷的脱氢硝化合成硝基芳香烯烃的新方法[J]. 有机化学, 2021, 41(1): 250-257.
Bing Mu, Junliang Wu, Guang'an Zhang. Alternative Approach for the Synthesis of Nitroaromatic Olefins via Dehydrogenative Nitration of Easily Available Arylethanes[J]. Chinese Journal of Organic Chemistry, 2021, 41(1): 250-257.
| Entry | Nitro source | [Cu] | Ligand | Oxidant | [Ag] | Additive | Solvent | Yield/% |
|---|---|---|---|---|---|---|---|---|
| 1 | NaNO 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 30 |
| 2 | NaNO 3 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 66 |
| 3 | KNO 3 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 56 |
| 4 | AgNO 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 39 |
| 5 | t BuNO 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 8 |
| 6 | Bi(NO 3) 3•5H 2O | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 57 |
| 7 | Ca(NO 3) 2•4H 2O | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 51 |
| 8 | Cd(NO 3) 2•4H 2O | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 54 |
| 9 | Co(NO 3) 2•6H 2O | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 25 |
| 10 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 80 |
| 11 | Pb(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 86 |
| 12 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | CF 3COOH | 22 |
| 13 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | Dioxane | 11 |
| 14 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DCE | 12 |
| 15 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMSO | 14 |
| 16 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMA | 8 |
| 17 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | NMP | NR |
| 18 | Ba(NO 3) 2 | CuBr | L 1 | Na 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 59 |
| 19 | Ba(NO 3) 2 | CuBr | L 1 | DDQ | CF 3COOAg | CF 3SO 3H | DMF | 10 |
| 20 | Ba(NO 3) 2 | CuBr | L 1 | t BuOO t Bu | CF 3COOAg | CF 3SO 3H | DMF | 6 |
| 21 | Ba(NO 3) 2 | CuBr | L 1 | t BuOOH | CF 3COOAg | CF 3SO 3H | DMF | NR |
| 22 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | AgNO 3 | CF 3SO 3H | DMF | 78 |
| 23 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CH 3COOAg | CF 3SO 3H | DMF | 75 |
| 24 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | Ag 2SO 4 | CF 3SO 3H | DMF | 73 |
| 25 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | Ag 2CO 3 | CF 3SO 3H | DMF | NR |
| 26 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3COOH | DMF | 42 |
| 27 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | TsOH | DMF | 16 |
| 28 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | t BuCOOH | DMF | 27 |
| 29 | Ba(NO 3) 2 | CuBr | L 2 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 75 |
| 30 | Ba(NO 3) 2 | CuBr | L 3 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 76 |
| 31 | Ba(NO 3) 2 | CuBr | L 4 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 64 |
| 32 | Ba(NO 3) 2 | CuBr | L 5 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 72 |
| 33 | Ba(NO 3) 2 | CuBr | L 6 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 79 |
| 34 | Ba(NO 3) 2 | CuOAc | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 92 (89) b |
| 35 | Ba(NO 3) 2 | CuI | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 80 |
| 36 | Ba(NO 3) 2 | (CF 3SO 3) 2Cu | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 80 |
| 37 | Ba(NO 3) 2 | [CF 3SO 3Cu] 2• C 6H 5CH 3 | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 82 |
| 38 | Ba(NO 3) 2 | Cu 2O | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 73 |
| 39 | Ba(NO 3) 2 | CuCl | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 76 |
| 40 | Ba(NO 3) 2 | CuBr 2 | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 77 |
| Entry | Nitro source | [Cu] | Ligand | Oxidant | [Ag] | Additive | Solvent | Yield/% |
|---|---|---|---|---|---|---|---|---|
| 1 | NaNO 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 30 |
| 2 | NaNO 3 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 66 |
| 3 | KNO 3 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 56 |
| 4 | AgNO 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 39 |
| 5 | t BuNO 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 8 |
| 6 | Bi(NO 3) 3•5H 2O | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 57 |
| 7 | Ca(NO 3) 2•4H 2O | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 51 |
| 8 | Cd(NO 3) 2•4H 2O | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 54 |
| 9 | Co(NO 3) 2•6H 2O | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 25 |
| 10 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 80 |
| 11 | Pb(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 86 |
| 12 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | CF 3COOH | 22 |
| 13 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | Dioxane | 11 |
| 14 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DCE | 12 |
| 15 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMSO | 14 |
| 16 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMA | 8 |
| 17 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | NMP | NR |
| 18 | Ba(NO 3) 2 | CuBr | L 1 | Na 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 59 |
| 19 | Ba(NO 3) 2 | CuBr | L 1 | DDQ | CF 3COOAg | CF 3SO 3H | DMF | 10 |
| 20 | Ba(NO 3) 2 | CuBr | L 1 | t BuOO t Bu | CF 3COOAg | CF 3SO 3H | DMF | 6 |
| 21 | Ba(NO 3) 2 | CuBr | L 1 | t BuOOH | CF 3COOAg | CF 3SO 3H | DMF | NR |
| 22 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | AgNO 3 | CF 3SO 3H | DMF | 78 |
| 23 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CH 3COOAg | CF 3SO 3H | DMF | 75 |
| 24 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | Ag 2SO 4 | CF 3SO 3H | DMF | 73 |
| 25 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | Ag 2CO 3 | CF 3SO 3H | DMF | NR |
| 26 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3COOH | DMF | 42 |
| 27 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | TsOH | DMF | 16 |
| 28 | Ba(NO 3) 2 | CuBr | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | t BuCOOH | DMF | 27 |
| 29 | Ba(NO 3) 2 | CuBr | L 2 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 75 |
| 30 | Ba(NO 3) 2 | CuBr | L 3 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 76 |
| 31 | Ba(NO 3) 2 | CuBr | L 4 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 64 |
| 32 | Ba(NO 3) 2 | CuBr | L 5 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 72 |
| 33 | Ba(NO 3) 2 | CuBr | L 6 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 79 |
| 34 | Ba(NO 3) 2 | CuOAc | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 92 (89) b |
| 35 | Ba(NO 3) 2 | CuI | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 80 |
| 36 | Ba(NO 3) 2 | (CF 3SO 3) 2Cu | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 80 |
| 37 | Ba(NO 3) 2 | [CF 3SO 3Cu] 2• C 6H 5CH 3 | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 82 |
| 38 | Ba(NO 3) 2 | Cu 2O | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 73 |
| 39 | Ba(NO 3) 2 | CuCl | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 76 |
| 40 | Ba(NO 3) 2 | CuBr 2 | L 1 | (NH 4) 2S 2O 8 | CF 3COOAg | CF 3SO 3H | DMF | 77 |
| [1] |
Reddy M.A.; Jain N.; Yada D.; Kishore C.; Reddy V.J.; Reddy P.S.; Addlagatta A.; Kalivendi S.V.; Sreedhar B. J. Med. Chem. 2011, 54, 6751.
|
| [2] |
Lu L.Q.; Chen J.R.; Xiao W.J. Acc. Chem. Res. 2012, 45, 1278.
|
| [3] |
Kaap S.; Quentin I.; Tamiru D.; Shaheen M.; Eger K.; Steinfelder H.J. Biochem. Pharmacol. 2003, 65, 603.
|
| [4] |
Uehara H.; Imashiro R.; Hernández-Torres G.; Barbas III, C.F.Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 20672.
|
| [5] |
Meah Y.; Massey V. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 10733.
|
| [6] |
Ishii T.; Fujioka S.; Sekiguchi Y.; Kotsuki H. J. Am. Chem. Soc. 2004, 126, 9558.
|
| [7] |
Huang H.B.; Jacobsen E.N. J. Am. Chem. Soc. 2006, 128, 7170.
|
| [8] |
Tripathi C.B.; Kayal S.; Mukherjee S. Org. Lett. 2012, 14, 3296.
|
| [9] |
Bai B.; Wang L.; Yang J.; Cai L.L.; Liu Q.J.; Xi, Gao. L.; Zhao, Z.W.; Mao, D.B.; Chen, Z.F. Chin. J. Org. Chem. 2019, 39, 1053. (in Chinese)
|
|
( 白冰, 王龙, 杨静, 蔡莉莉, 刘前进, 席高磊, 赵志伟, 毛多斌, 陈芝飞, 有机化学, 2019, 39, 1053.).
|
|
| [10] |
Luo S.P.; Wang L.P.; Yue H.D.; Le Z.G.; Yang W.L.; Xu D.Q.; Xu Z.Y. Acta Chim Sinica. 2006, 64, 1483.
|
| [11] |
Evans D.A.; Mito S.; Seidel D. J. Am. Chem. Soc. 2007, 129, 11583.
|
| [12] |
March J. Advanced Organic Chemistry, 3rd
|
| [13] |
Albrecht L.; Dickmeiss G.; Acosta F.C.; Rodríguez-Escrich C.; Davis R.L.; Jorgensen K.A. J. Am. Chem. Soc. 2012, 134, 2543.
|
| [14] |
Liu Y.K.; Nappi M.; Arceo E.; Vera S.; Melchiorre P. J. Am. Chem. Soc. 2011, 133, 15212.
|
| [15] |
Arai T.; Mishiro A.; Yokoyama N.; Suzuki K.; Sato H. J. Am. Chem. Soc. 2010, 132, 5338.
|
| [16] |
Denmark S.E.; Thorarensen A. Chem. Rev. 1996, 96, 137.
|
| [17] |
Yan L.J.; Xu H.; Wang Y.; Dong J.W.; Wang Y.C. Chin. J. Org. Chem. 2020, 40, 284. (in Chinese)
|
|
( 严丽君, 徐菡, 王艳, 董建伟, 王永超, 有机化学, 2020, 40, 284.).
|
|
| [18] |
Basavaiah D.; Reddy B.S.; Badsara S.S. Chem. Rev. 2010, 110, 5447.
|
| [19] |
Nair D.K.; Mobin S.M.; Namboothiri I.N.N. Org. Lett. 2012, 14, 4580.
|
| [20] |
Quan X.J.; Ren Z.H.; Wang Y.Y.; Guan Z.H. Org. Lett. 2014, 16, 5728.
|
| [21] |
Chen Y.F.; Nie G.; Zhang Q.; Ma S.; Li H.; Hu Q.Q. Org. Lett. 2015, 17, 1118.
|
| [22] |
Kurti L.; Czako B. Strategic Applications of Named Reactions in Organic Synthesis, Elsevier Academic Press, London , 2005.
|
| [23] |
Fioravanti S.; Pellacani L.; Tardella P.A.; Vergari M.C. Org. Lett. 2008, 10, 1449.
|
| [24] |
Hassner A.; Kropp J.E.; Kent G.J. J. Org. Chem. 1969, 34, 2628.
|
| [25] |
Ranganathan S.; Kar S.K. J. Org. Chem. 1970, 35, 3962.
|
| [26] |
Corey E.J.; Estreicher H. J. Am. Chem. Soc. 1978, 100, 6294.
|
| [27] |
Barluenga J.; Rodríguez M.A.; Campos P.J.; Asensio G. J. Chem. Soc., Chem. Commun. 1987, 1491.
|
| [28] |
Barluenga J.; Rodríguez M.A.; Campos P.J. J. Chem. Soc., Perkin Trans. 1 1990, 2807.
|
| [29] |
Sy W.W.; By A.W. Tetrahedron Lett. 1985, 26, 1193.
|
| [30] |
Jew S.S.; Kim H.D.; Cho Y.S.; Cook C.H. Chem. Lett. 1986, 15, 1747.
|
| [31] |
Hwu J.R.; Chen K.L.; Ananthan S.; Patel H.V. Organometallics 1996, 15, 499.
|
| [32] |
Ghosh D.; Nichols D.E. Synthesis 1996, 195.
|
| [33] |
Suzuki H.; Mori T. J. Org. Chem. 1997, 62, 6498.
|
| [34] |
Mukaiyama T.; Hata E.; Yamada T. Chem. Lett. 1995, 24, 505.
|
| [35] |
Hata E.; Yamada T.; Mukaiyama T. Bull. Chem. Soc. Jpn. 1995, 68, 3629.
|
| [36] |
Jovel I.; Prateeptongkum S.; Jackstell R.; Vogl N.; Weckbecker C.; Beller M. Adv. Synth. Catal. 2008, 350, 2493.
|
| [37] |
Varma R.S.; Naicker K.P.; Liesent P.J. Tetrahedron Lett. 1998, 39, 3977.
|
| [38] |
Manna S.; Jana S.; Saboo T.; Maji A.; Maiti D. Chem. Commun. 2013, 49, 5286.
|
| [39] |
Rokade B.V.; Prabhu K.R. Org. Biomol. Chem. 2013, 11, 6713.
|
| [40] |
Das J.P.; Sinha P.; Roy S. Org. Lett. 2002, 4, 3055.
|
| [41] |
Baruah D.; Pahari P.; Konwar D. Tetrahedron Lett. 2015, 56, 2418.
|
| [42] |
Yang Z.; Li J.; Hua J.; Yang T.; Yi J.M.; Zhou C.S. Synlett 2017, 28, 1079.
|
| [43] |
Luo Z.G.; Xu F.; Fang Y.Y.; Liu P.; Xu X.M.; Feng C.T.; Li Z.; He J. Res. Chem. Intermed. 2016, 42, 6079.
|
| [44] |
Maity S.; Manna S.; Rana S.; Naveen T.; Mallick A.; Maiti D. J. Am. Chem. Soc. 2013, 135, 3355.
|
| [45] |
Naveen T.; Maity S.; Sharma U.; Maiti D. J. Org. Chem. 2013, 78, 5949.
|
| [46] |
Maity S.; Naveen T.; Sharma U.; Maiti D. Org. Lett. 2013, 15, 3384.
|
| [47] |
Zhao A.; Jiang Q.; Jia J.; Xu B.; Liu Y.F.; Zhang M.Z.; Liu Q.; Luo W.P.; Guo C.C. Tetrahedron Lett. 2016, 57, 80.
|
| [48] |
Whiting K.; Carmona L.G.; Sousa T. Renewable Sustainable Energy Rev. 2017, 76, 202.
|
| [49] |
Degnan T.F. J. Catal. 2003, 216, 32.
|
| [50] |
Grant J.T.; Venegas J.M.; McDermott W.P.; Hermans I. Chem. Rev. 2018, 118, 2769.
|
| [51] |
Kumar A.; Bhatti T.M.; Goldman A.S. Chem. Rev. 2017, 117, 12357.
|
| [52] |
Wang Y.L.; Qian L.; Huang Z.D.; Liu G.X.; Huang Z. Chin. J. Chem. 2020, 38, 837.
|
| [53] |
Sathyamoorthi S.; Banerjee S. ChemistrySelect 2017, 2, 10678.
|
| [54] |
Sathyamoorthi S.; Du Bois, J. Org. Lett. 2016, 18, 6308.
|
| [55] |
Banerjee S.; Sathyamoorthi S.; Du Bois J.; Zare R.N. Chem. Sci. 2017, 8, 7003.
|
| [56] |
Manna S.; Antonchick A.P. Chem. -Eur. J. 2017, 23, 7825.
|
| [57] |
Burkhard C.A.; Brown J.F., Jr. US 2867669, 1959.
|
| [58] |
Tang X.J.; Dolbier Jr.W.R. Angew. Chem. Int. Ed. 2015, 54, 4246.
|
| [59] |
Ambala S.; Singh R.; Singh M.; Cham P.S.; Gupta R.; Munagala G.; Yempalla K.R.; Vishwakarma R.A.; Singh P.P. RSC Adv. 2019, 9, 30428.
|
| [60] |
Huie R.E.; Clifton C.L.; Kafafi S.A. J. Phys. Chem. 1991, 95, 9336.
|
| [61] |
Huie R.E.; Clifton C.L. J. Phys. Chem. 1990, 94, 8561.
|
| [62] |
Liu Y.; Wang Q.L.; Chen Z.; Zhou Q.; Zhou C.S.; Xiong B.Q.; Zhang P.L.; Yang C.A.; Tang K.W. Org. Biomol. Chem. 2019, 17, 1365.
|
| [63] |
Manna S.; Jana S.; Saboo T.; Maji A.; Maiti D. Chem. Commun. 2013, 49, 5286.
|
| [64] |
Gross Z.; Hoz S. J. Am. Chem. Soc. 1988, 110, 7489.
|
| [65] |
Hsieh T.H.H.; Dong V.M. Tetrahedron 2009, 65, 3062.
|
| [66] |
Zhao A.; Jiang Q.; Jia J.; Xu, Bin.; Liu, Y.F.; Zhang, M.Z.; Liu, Q.; Luo, W.P.; Guo, C.C. Tetrahedron Lett. 2016, 57, 80.
|
| [67] |
Ambala S.; Singh R.; Singh M.; Cham P.S.; Gupta R.; Munagala G.; Yempalla K.R.; Vishwakarma R.A.; Singh P.P. RSC Adv. 2019, 9, 30428.
|
| [1] | 易荣楠, 刘冬娴, 贺江南, 赵明明, 许新华. 过硫酸铵促进喹喔啉-2(1H)-酮三氟甲基化反应[J]. 有机化学, 2021, 41(8): 3285-3291. |
| [2] | 江晓莉, 戴伟, 赵佳佳, 石枫. 布朗斯特酸催化下邻羟基苯乙烯与吲哚的反应——1,1-二芳基乙烷类化合物的合成[J]. 有机化学, 2016, 36(5): 1014-1020. |
| [3] | 陈翠, 谭丽泉, 邱会华. 乙酰乙酰芳胺类化合物导向的五取代1,4-二氢吡啶衍生物的合成[J]. 有机化学, 2014, 34(8): 1673-1676. |
| [4] | 陈芬儿,严琼娇,彭作中,张道明,傅晗,刘继东. 四正丁基过硫酸铵氧化裂解2,4-二硝基苯腙衍生物的反应研究[J]. 有机化学, 2000, 20(1): 116-121. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||