

利用微波辅助[3+3]环化反应合成稠合吡啶衍生物
收稿日期: 2021-02-04
修回日期: 2021-02-10
网络出版日期: 2021-02-26
基金资助
国家自然科学基金(21971090); 江苏省品牌专业基金资助项目
Synthesis of Fused Pyridines via Microwave-Assisted [3+3] Cyclization
Received date: 2021-02-04
Revised date: 2021-02-10
Online published: 2021-02-26
Supported by
National Natural Science Foundation of China(21971090); Top-notch Academic Programs Project of Jiangsu Higher Education Institutions
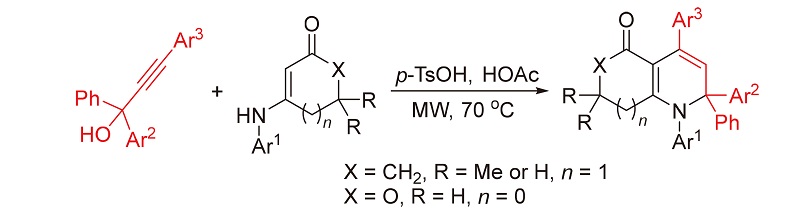
报道了一类新型的微波辅助对甲基苯磺酸促进的[3+3]环化反应. 利用烯胺酮或烯胺内酯可作为1,3-双亲核试剂及炔丙醇可作为1,3-双亲电试剂的特性, 使其在微波辐射及对甲苯磺酸促进条件下于冰醋酸中在70 ℃反应, 实现了[3+3]环化反应, 分别区域选择性地合成了2,2-二芳基取代四氢喹啉-5(1H)-酮衍生物和2,2-二芳基取代二氢呋喃并[3,4-b]吡啶-5-酮衍生物, 产率良好. 该反应利用微波合成技术促使反应, 在短时间内完成(30 min), 唯一副产物为水. 此外, 该方法具有原料简单易得、操作简单及底物普适性广等优点, 从而为具有潜在应用价值的稠合吡啶骨架的构建提供了一种绿色、经济且高效的合成策略, 符合绿色化学理念.
关键词: 微波合成; [3+3]环化; 喹啉-5(1H)-酮衍生物; 呋喃并[3,4-b]吡啶-5-酮衍生物; 绿色化学
吴亚男 , 杜建宇 , 郝文娟 , 姜波 . 利用微波辅助[3+3]环化反应合成稠合吡啶衍生物[J]. 有机化学, 2021 , 41(4) : 1563 -1571 . DOI: 10.6023/cjoc202102018
A new microwave-assisted p-TsOH-promoted [3+3] cyclization was developed. By using the characteristics of enaminones or enamino lactones as 1,3-dinucleaphilic reagents and propargyl alcohols as 1,3-electrophilic reagents, p-TsOH- promoted [3+3] cyclization of these substrates at 70 ℃ was carried out in acetic acid under microwave irradiation, regioselectively affording 2,2-diaryl-substituted tetrahydroquinoline-5(1H)-ones and 2,2-diaryl-substituted dihydrofuro[3,4-b]pyridin- 5-ones in good yields. The reaction can be completed within a short period (30 min) by microwave synthetic technology, in which water was the sole by-product. This method features simple and available starting materials, simple operation and wide substrate scope, and provides a green, economic, and efficient synthetic strategy for the construction of fused pyridine skeleton with potential application, which is consistent with the concept of green chemistry.

| [1] | (a) Gan, L.; Wei, L.; Wan, J.P. ChemistrySelect 2020, 5,7822. |
| [1] | (b) Xiong, J.; Zhong, G.F.; Liu, Y.-Y. Adv. Synth. Catal. 2019, 361,550. |
| [1] | (c) Wang, L.; Shi, L.-X.; Liu, L.; Li, Z.-X.; Xu, T.; Hao, W.-J.; Li, G.; Tu, S.-J.; Jiang, B. J. Org. Chem. 2017, 82,3605. |
| [1] | (d) Wu, Y.; Lin, Y.W.; He, W.M. Chin. Chem. Lett. 2020, 31,2999. |
| [1] | (e) Li, W.; Shu, L.; Wang, Q.; Li, G.Y.; Shan, Y. Chin. J. Org. Chem. 2019, 39,1976. (in Chinese) |
| [1] | ( 李蔚, 宿亮, 汪秋安, 李高阳, 单杨, 有机化学, 2019, 39,1976.) |
| [2] | (a) Bose, A.K.; Manhas, M.S.; Ghosh, M.; Shah, M.; Raju, V.S.; Bari, S.S.; Newaz, S.N.; Banik, B.K.; Chaudhary, A.G.; Barakat, K.J. J. Org. Chem. 1991, 56,6968. |
| [2] | (b) Baghurst, D.R.; Mingos, D.M. P. J. Organomet. Chem. 1990, 384,57. |
| [2] | (c) Raner, K.D.; Strauss, C.R. J. Org. Chem. 1992, 57,6231. |
| [2] | (d) Caddick, S. Tetrahedron 1995, 51,10403. |
| [3] | (a) Sheng, J.; Wang, Y.; Su, X. Angew. Chem., Int. Ed. 2017, 56,4824. |
| [3] | (b) Lin, W.; Zhuang, C. W.; Hu, X. X. Chin. J. Org. Chem. 2020, 40,408. (in Chinese) |
| [3] | ( 林伟, 庄苍伟, 胡秀秀, 有机化学, 2020, 40,408.) |
| [3] | (c) Zeng, R.; Shan, C.; Liu, M. Org. Lett. 2019, 21,2312. |
| [3] | (d) Lan, X.-C.; Chen, T.-T.; Zhao, Y.; Wu, Y.; Wang, J.; Tu, S.-J.; Jiang, B.; Hao, W.-J. Tetrahedron Lett. 2017, 58,1519. |
| [3] | (e) Wang, A.-F.; Zhou, P.; Zhu, Y.-L.; Hao, W.J.; Li, G.; Tu, S.-J.; Jiang, B. Chem. Commun. 2017, 53,3369. |
| [3] | (f) Jiang, B.; Zhang, T.-S.; Fu, R.; Hao, W.-J.; Wang, S.-L.; Tu, S.-J. Tetrahedron 2016, 72,5652. |
| [3] | (g) Fan, W.; Li, Y.-R.; Li, Q.; Jiang, B.; Li, G. Tetrahedron 2016, 72,4867. |
| [3] | (h) Hao, W.J.; Zhou, P.; Wu, F.Y.; Jiang, B.; Tu, S.J.; Li, G. Eur. J. Org. Chem. 2016, 2016,1968. |
| [4] | Kumar, D.; Jain, S.K. Curr. Med. Chem. 2016, 23,4338. |
| [5] | (a) Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Gharanjig, K. J. Hazand. Mater. 2007, 145,65. |
| [5] | (b) Amuti, K.; Trombini, A.; Giammamusti, L.; Sbriscia, C.; Harder, H.; Gabard, J. Brighton Crop Prot. Conf.--Weeds 1997, 1,59. |
| [5] | (c) Ma, Q.; Zhang, X.; Qu, Y. Front. Microbiol. 2018, 9,2625. |
| [6] | (a) Chen, D.; Su, S.J.; Cao, Y. J. Mater. Chem. 2014, 2,9565. |
| [6] | (b) Domcke, W.; Ehrmaier, J.; Sobolewski, A.L. ChemPhotoChem 2019, 3,10. |
| [7] | (a) Bhunia, A.; Biju, A.T. Synlett 2014, 25,608. |
| [7] | (b) Kuhl, O. Coord. Chem. Rev. 2004, 248,411. |
| [8] | (a) Cowley, R.; Leung, S.; Fisher, N.; Al-Helal, M.; Berry, N.G.; Lawrenson, A.S.; Sharma, R.; Shone, A.E.; Ward, S.A.; Biagini, G.A.; O'Neill, P.M. MedChemComm 2012, 3,39. |
| [8] | (b) Da Cruz, F.P.; Martin, C.; Buchholz, K.; Lafuente-Monasterio, M.J.; Rodrigues, T.; Sonnichsen, B.; Moreira, R.; Gamo, F.J.; Marti, M.; Mota, M.M.; Hannus, M.; Prudencio, M. J. Infect. Dis. 2012, 205,1278. |
| [9] | Reynolds, K.A.; Loughlin, W.A.; Young, D.J. Mini-Rev. Med. Chem. 2013, 13,730. |
| [10] | (a) Bisacchi, G.S. J. Med. Chem. 2015, 58,4874. |
| [10] | (b) Sissi, C.; Palumbo, M. Curr. Med. Chem.: Anti-Cancer Agents 2003, 3,439. |
| [11] | (a) Oehldrich, J.; Cook, J.M. J. Org. Chem. 1977, 42,889. |
| [11] | (b) Yan, W.T. Org. Biomol. Chem. 2018, 16,9440. |
| [12] | (a) Sarkar, R.; Mukhopadhyay, C. Tetrahedron Lett. 2014, 55,2618. |
| [12] | (b) Sarkar, R.; Mukhopadhyay, C. Tetrahedron Lett. 2018, 59,3069. |
| [13] | Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2011, 2011,6952. |
| [14] | Ahmed, N.S.; Badahdah, K.O.; Qassar, H.M. Med. Chem. Res. 2017, 26,1201. |
| [15] | (a) Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2012, 2012,1976. |
| [15] | (b) Debnath, K.; Pramanik, A. Tetrahedron Lett. 2015, 56,1654. |
| [15] | (c) Nikoofar, K.; Khani, S. Catal. Lett. 2018, 148,1651. |
| [15] | (d) Zhu, D.; Sun, J.; Yan, C.-G. J. Heterocycl. Chem. 2016, 53,583. |
| [16] | (a) Li, M.; Wang, R.; Hao, W.; Jiang, B. Chin. J. Org. Chem. 2020, 40,1540. (in Chinese) |
| [16] | ( 李梦帆, 王榕, 郝文娟, 姜波, 有机化学, 2020, 40,1540.) |
| [16] | (b) Wang, R.; Xu, L.; Lu, Y.; Jiang, B.; Hao, W. Chin. J. Org. Chem. 2021, 41,1582. (in Chinese) |
| [16] | ( 王榕, 徐立晨, 卢逸, 姜波, 郝文娟, 有机化学, 2021, 41,1582.) |
| [16] | (c) Li, Q.; Li, M.; Shi, S.; Ji, X.; He, C.; Jiang, B.; Hao, W. Chin. J. Org. Chem. 2020, 40,384. (in Chinese) |
| [16] | ( 李庆雪, 李梦伟, 时绍青, 季晓霜, 何春兰, 姜波, 郝文娟, 有机化学, 2020, 40,384.) |
| [16] | (d) Wang, D.; Wang, S.-C.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Chin. J. Chem. 2021, 39,106. |
| [16] | (e) Zuo, H.-D.; Hao, W.-J.; Zhu, C.-F.; Guo, C.; Tu, S.-J.; Jiang, B. Org. Lett. 2020, 22,4471. |
| [17] | (a) Gan, L.; Yu, Q.; Liu, Y.Y.; Wan, J.P. J. Org. Chem. 2021, 86,1231. |
| [17] | (b) Fu, L.Q.; Xu, Z.R.; Wan, J.P.; Liu, Y.Y. Org. Lett. 2020, 22,9518. |
| [17] | (c) Yu, Q.; Liu, Y.Y.; Wan, J.P. Org. Chem. Front. 2020, 7,2770. |
| [17] | (d) Luo, T.; Wan, J.P.; Liu, Y.Y. Org. Chem. Front. 2020, 7,1107. |
| [17] | (e) Wang, G.D.; Guo, Y.H.; Wan, J.P. Chin. J. Org. Chem. 2020, 40,645. (in Chinese) |
| [17] | ( 王国栋, 郭艳辉, 万结平, 有机化学, 2020, 40,645.) |
| [17] | (f) Liu, Y.Y.; Xiong, J.; Wan, J.P. Adv. Synth. Catal. 2020, 362,877. |
| [18] | (a) Gui, Q.W.; He, X.L.; Wang, W.J.; Zhou, H.L.; Dong, Y.M.; Wang, N.Q.; Tang, J.X.; Cao, Z.; He, W.M. Green Chem. 2020, 22,118. |
| [18] | (b) Wang, Z.; He, W.M. Chin. J. Org. Chem. 2019, 39,3594. (in Chinese) |
| [18] | ( 王峥, 何卫民, 有机化学, 2019, 39,3594.) |
| [18] | (c) Wu, Y.Q.; He, W.M. Chin. J. Org. Chem. 2020, 40,2597. (in Chinese) |
| [18] | ( 吴燕, 何卫民, 有机化学, 2020, 40,2597.) |
| [18] | (d) Zhang, P.; Shi, H.N.; Zhang, T.S.; Cai, P.J.; Jiang, B.; Tu, S.J. Chin. J. Org. Chem. 2020, 40,423. (in Chinese) |
| [18] | ( 张萍, 石浩楠, 张天舒, 蔡佩君, 姜波, 屠树江, 有机化学, 2020, 40,423.) |
| [19] | (a) Bandgar, B.P.; Patil, S.A.; Korbad, B.L.; Bandgar, S.B.; Hote, B.S. Aust. J. Chem. 2008, 61,552. |
| [19] | (b) Matam, S.K. P.; Perumal, M.S. ChemistrySelect 2017, 2,2363. |
| [19] | (c) da Rocha Pissurno, A.P.; da Silva de Laurentiz, R. Synth. Commun. 2017, 47,1874. |
| [19] | (d) Takefumi, M.; Naoki, T.; Takafumi, N.; Yumi, T. Heterocycles 1988, 27,1907. |
/
| 〈 |
|
〉 |