有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1563-1571.DOI: 10.6023/cjoc202102018 上一篇 下一篇
研究论文
吴亚男a,b,*( ), 杜建宇b, 郝文娟b, 姜波b,*(
), 杜建宇b, 郝文娟b, 姜波b,*( )
)
收稿日期:2021-02-04
修回日期:2021-02-10
发布日期:2021-02-26
通讯作者:
吴亚男, 姜波
基金资助:
Yanan Wua,b,*( ), Jianyu Dub, Wenjuan Haob, Bo Jiangb,*(
), Jianyu Dub, Wenjuan Haob, Bo Jiangb,*( )
)
Received:2021-02-04
Revised:2021-02-10
Published:2021-02-26
Contact:
Yanan Wu, Bo Jiang
About author:Supported by:文章分享
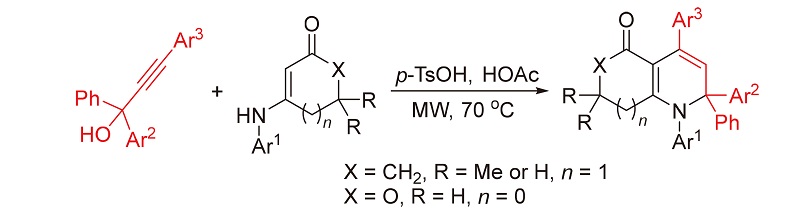
报道了一类新型的微波辅助对甲基苯磺酸促进的[3+3]环化反应. 利用烯胺酮或烯胺内酯可作为1,3-双亲核试剂及炔丙醇可作为1,3-双亲电试剂的特性, 使其在微波辐射及对甲苯磺酸促进条件下于冰醋酸中在70 ℃反应, 实现了[3+3]环化反应, 分别区域选择性地合成了2,2-二芳基取代四氢喹啉-5(1H)-酮衍生物和2,2-二芳基取代二氢呋喃并[3,4-b]吡啶-5-酮衍生物, 产率良好. 该反应利用微波合成技术促使反应, 在短时间内完成(30 min), 唯一副产物为水. 此外, 该方法具有原料简单易得、操作简单及底物普适性广等优点, 从而为具有潜在应用价值的稠合吡啶骨架的构建提供了一种绿色、经济且高效的合成策略, 符合绿色化学理念.
吴亚男, 杜建宇, 郝文娟, 姜波. 利用微波辅助[3+3]环化反应合成稠合吡啶衍生物[J]. 有机化学, 2021, 41(4): 1563-1571.
Yanan Wu, Jianyu Du, Wenjuan Hao, Bo Jiang. Synthesis of Fused Pyridines via Microwave-Assisted [3+3] Cyclization[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1563-1571.
| Entry | Promotor (equiv.) | Solvent | Temp./℃ | Yieldb/% |
|---|---|---|---|---|
| 1 | p-TsOH (1.0) | Acetone | 100 | N.R. |
| 2 | p-TsOH (1.0) | DCE | 100 | N.R. |
| 3 | p-TsOH (1.0) | DCM | 100 | Trace |
| 4 | p-TsOH (1.0) | MeCN | 100 | 30 |
| 5 | p-TsOH (1.0) | Toluene | 100 | Trace |
| 6 | p-TsOH (1.0) | EtOH | 100 | Trace |
| 7 | p-TsOH (1.0) | HOAc | 100 | 58 |
| 8 | p-TsOH (0.5) | HOAc | 100 | 45 |
| 9 | p-TsOH (2.0) | HOAc | 100 | 55 |
| 10 | BF3?Et2O (1.0) | HOAc | 100 | 37 |
| 11 | TFA (1.0) | HOAc | 100 | 21 |
| 12 | TfOH (1.0) | HOAc | 100 | Trace |
| 13 | p-TsOH (1.0) | HOAc | 90 | 61 |
| 14 | p-TsOH (1.0) | HOAc | 80 | 65 |
| 15 | p-TsOH (1.0) | HOAc | 70 | 70 |
| 16 | p-TsOH (1.0) | HOAc | 60 | 60 |
| Entry | Promotor (equiv.) | Solvent | Temp./℃ | Yieldb/% |
|---|---|---|---|---|
| 1 | p-TsOH (1.0) | Acetone | 100 | N.R. |
| 2 | p-TsOH (1.0) | DCE | 100 | N.R. |
| 3 | p-TsOH (1.0) | DCM | 100 | Trace |
| 4 | p-TsOH (1.0) | MeCN | 100 | 30 |
| 5 | p-TsOH (1.0) | Toluene | 100 | Trace |
| 6 | p-TsOH (1.0) | EtOH | 100 | Trace |
| 7 | p-TsOH (1.0) | HOAc | 100 | 58 |
| 8 | p-TsOH (0.5) | HOAc | 100 | 45 |
| 9 | p-TsOH (2.0) | HOAc | 100 | 55 |
| 10 | BF3?Et2O (1.0) | HOAc | 100 | 37 |
| 11 | TFA (1.0) | HOAc | 100 | 21 |
| 12 | TfOH (1.0) | HOAc | 100 | Trace |
| 13 | p-TsOH (1.0) | HOAc | 90 | 61 |
| 14 | p-TsOH (1.0) | HOAc | 80 | 65 |
| 15 | p-TsOH (1.0) | HOAc | 70 | 70 |
| 16 | p-TsOH (1.0) | HOAc | 60 | 60 |
| [1] |
(a) Gan, L.; Wei, L.; Wan, J.P. ChemistrySelect 2020, 5,7822.
pmid: 28296402 |
|
(b) Xiong, J.; Zhong, G.F.; Liu, Y.-Y. Adv. Synth. Catal. 2019, 361,550.
pmid: 28296402 |
|
|
(c) Wang, L.; Shi, L.-X.; Liu, L.; Li, Z.-X.; Xu, T.; Hao, W.-J.; Li, G.; Tu, S.-J.; Jiang, B. J. Org. Chem. 2017, 82,3605.
doi: 10.1021/acs.joc.7b00129 pmid: 28296402 |
|
|
(d) Wu, Y.; Lin, Y.W.; He, W.M. Chin. Chem. Lett. 2020, 31,2999.
pmid: 28296402 |
|
|
(e) Li, W.; Shu, L.; Wang, Q.; Li, G.Y.; Shan, Y. Chin. J. Org. Chem. 2019, 39,1976. (in Chinese)
pmid: 28296402 |
|
|
( 李蔚, 宿亮, 汪秋安, 李高阳, 单杨, 有机化学, 2019, 39,1976.)
pmid: 28296402 |
|
| [2] |
(a) Bose, A.K.; Manhas, M.S.; Ghosh, M.; Shah, M.; Raju, V.S.; Bari, S.S.; Newaz, S.N.; Banik, B.K.; Chaudhary, A.G.; Barakat, K.J. J. Org. Chem. 1991, 56,6968.
|
|
(b) Baghurst, D.R.; Mingos, D.M. P. J. Organomet. Chem. 1990, 384,57.
|
|
|
(c) Raner, K.D.; Strauss, C.R. J. Org. Chem. 1992, 57,6231.
|
|
|
(d) Caddick, S. Tetrahedron 1995, 51,10403.
|
|
| [3] |
(a) Sheng, J.; Wang, Y.; Su, X. Angew. Chem., Int. Ed. 2017, 56,4824.
pmid: 30900459 |
|
(b) Lin, W.; Zhuang, C. W.; Hu, X. X. Chin. J. Org. Chem. 2020, 40,408. (in Chinese)
pmid: 30900459 |
|
|
( 林伟, 庄苍伟, 胡秀秀, 有机化学, 2020, 40,408.)
pmid: 30900459 |
|
|
(c) Zeng, R.; Shan, C.; Liu, M. Org. Lett. 2019, 21,2312.
pmid: 30900459 |
|
|
(d) Lan, X.-C.; Chen, T.-T.; Zhao, Y.; Wu, Y.; Wang, J.; Tu, S.-J.; Jiang, B.; Hao, W.-J. Tetrahedron Lett. 2017, 58,1519.
pmid: 30900459 |
|
|
(e) Wang, A.-F.; Zhou, P.; Zhu, Y.-L.; Hao, W.J.; Li, G.; Tu, S.-J.; Jiang, B. Chem. Commun. 2017, 53,3369.
pmid: 30900459 |
|
|
(f) Jiang, B.; Zhang, T.-S.; Fu, R.; Hao, W.-J.; Wang, S.-L.; Tu, S.-J. Tetrahedron 2016, 72,5652.
pmid: 30900459 |
|
|
(g) Fan, W.; Li, Y.-R.; Li, Q.; Jiang, B.; Li, G. Tetrahedron 2016, 72,4867.
pmid: 30900459 |
|
|
(h) Hao, W.J.; Zhou, P.; Wu, F.Y.; Jiang, B.; Tu, S.J.; Li, G. Eur. J. Org. Chem. 2016, 2016,1968.
pmid: 30900459 |
|
| [4] |
Kumar, D.; Jain, S.K. Curr. Med. Chem. 2016, 23,4338.
doi: 10.2174/0929867323666160809093930 pmid: 27516198 |
| [5] |
(a) Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Gharanjig, K. J. Hazand. Mater. 2007, 145,65.
pmid: 30443243 |
|
(b) Amuti, K.; Trombini, A.; Giammamusti, L.; Sbriscia, C.; Harder, H.; Gabard, J. Brighton Crop Prot. Conf.--Weeds 1997, 1,59.
pmid: 30443243 |
|
|
(c) Ma, Q.; Zhang, X.; Qu, Y. Front. Microbiol. 2018, 9,2625.
doi: 10.3389/fmicb.2018.02625 pmid: 30443243 |
|
| [6] |
(a) Chen, D.; Su, S.J.; Cao, Y. J. Mater. Chem. 2014, 2,9565.
|
|
(b) Domcke, W.; Ehrmaier, J.; Sobolewski, A.L. ChemPhotoChem 2019, 3,10.
|
|
| [7] |
(a) Bhunia, A.; Biju, A.T. Synlett 2014, 25,608.
|
|
(b) Kuhl, O. Coord. Chem. Rev. 2004, 248,411.
|
|
| [8] |
(a) Cowley, R.; Leung, S.; Fisher, N.; Al-Helal, M.; Berry, N.G.; Lawrenson, A.S.; Sharma, R.; Shone, A.E.; Ward, S.A.; Biagini, G.A.; O'Neill, P.M. MedChemComm 2012, 3,39.
pmid: 22396598 |
|
(b) Da Cruz, F.P.; Martin, C.; Buchholz, K.; Lafuente-Monasterio, M.J.; Rodrigues, T.; Sonnichsen, B.; Moreira, R.; Gamo, F.J.; Marti, M.; Mota, M.M.; Hannus, M.; Prudencio, M. J. Infect. Dis. 2012, 205,1278.
doi: 10.1093/infdis/jis184 pmid: 22396598 |
|
| [9] |
Reynolds, K.A.; Loughlin, W.A.; Young, D.J. Mini-Rev. Med. Chem. 2013, 13,730.
|
| [10] |
(a) Bisacchi, G.S. J. Med. Chem. 2015, 58,4874.
doi: 10.1021/jm501881c pmid: 14529452 |
|
(b) Sissi, C.; Palumbo, M. Curr. Med. Chem.: Anti-Cancer Agents 2003, 3,439.
pmid: 14529452 |
|
| [11] |
(a) Oehldrich, J.; Cook, J.M. J. Org. Chem. 1977, 42,889.
pmid: 30515497 |
|
(b) Yan, W.T. Org. Biomol. Chem. 2018, 16,9440.
doi: 10.1039/c8ob02701c pmid: 30515497 |
|
| [12] |
(a) Sarkar, R.; Mukhopadhyay, C. Tetrahedron Lett. 2014, 55,2618.
|
|
(b) Sarkar, R.; Mukhopadhyay, C. Tetrahedron Lett. 2018, 59,3069.
|
|
| [13] |
Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2011, 2011,6952.
|
| [14] |
Ahmed, N.S.; Badahdah, K.O.; Qassar, H.M. Med. Chem. Res. 2017, 26,1201.
|
| [15] |
(a) Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Eur. J. Org. Chem. 2012, 2012,1976.
|
|
(b) Debnath, K.; Pramanik, A. Tetrahedron Lett. 2015, 56,1654.
|
|
|
(c) Nikoofar, K.; Khani, S. Catal. Lett. 2018, 148,1651.
|
|
|
(d) Zhu, D.; Sun, J.; Yan, C.-G. J. Heterocycl. Chem. 2016, 53,583.
|
|
| [16] |
(a) Li, M.; Wang, R.; Hao, W.; Jiang, B. Chin. J. Org. Chem. 2020, 40,1540. (in Chinese)
pmid: 32402201 |
|
( 李梦帆, 王榕, 郝文娟, 姜波, 有机化学, 2020, 40,1540.)
pmid: 32402201 |
|
|
(b) Wang, R.; Xu, L.; Lu, Y.; Jiang, B.; Hao, W. Chin. J. Org. Chem. 2021, 41,1582. (in Chinese)
pmid: 32402201 |
|
|
( 王榕, 徐立晨, 卢逸, 姜波, 郝文娟, 有机化学, 2021, 41,1582.)
pmid: 32402201 |
|
|
(c) Li, Q.; Li, M.; Shi, S.; Ji, X.; He, C.; Jiang, B.; Hao, W. Chin. J. Org. Chem. 2020, 40,384. (in Chinese)
pmid: 32402201 |
|
|
( 李庆雪, 李梦伟, 时绍青, 季晓霜, 何春兰, 姜波, 郝文娟, 有机化学, 2020, 40,384.)
pmid: 32402201 |
|
|
(d) Wang, D.; Wang, S.-C.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Chin. J. Chem. 2021, 39,106.
pmid: 32402201 |
|
|
(e) Zuo, H.-D.; Hao, W.-J.; Zhu, C.-F.; Guo, C.; Tu, S.-J.; Jiang, B. Org. Lett. 2020, 22,4471.
doi: 10.1021/acs.orglett.0c01470 pmid: 32402201 |
|
| [17] |
(a) Gan, L.; Yu, Q.; Liu, Y.Y.; Wan, J.P. J. Org. Chem. 2021, 86,1231.
pmid: 33269937 |
|
(b) Fu, L.Q.; Xu, Z.R.; Wan, J.P.; Liu, Y.Y. Org. Lett. 2020, 22,9518.
doi: 10.1021/acs.orglett.0c03548 pmid: 33269937 |
|
|
(c) Yu, Q.; Liu, Y.Y.; Wan, J.P. Org. Chem. Front. 2020, 7,2770.
pmid: 33269937 |
|
|
(d) Luo, T.; Wan, J.P.; Liu, Y.Y. Org. Chem. Front. 2020, 7,1107.
pmid: 33269937 |
|
|
(e) Wang, G.D.; Guo, Y.H.; Wan, J.P. Chin. J. Org. Chem. 2020, 40,645. (in Chinese)
pmid: 33269937 |
|
|
( 王国栋, 郭艳辉, 万结平, 有机化学, 2020, 40,645.)
pmid: 33269937 |
|
|
(f) Liu, Y.Y.; Xiong, J.; Wan, J.P. Adv. Synth. Catal. 2020, 362,877.
pmid: 33269937 |
|
| [18] |
(a) Gui, Q.W.; He, X.L.; Wang, W.J.; Zhou, H.L.; Dong, Y.M.; Wang, N.Q.; Tang, J.X.; Cao, Z.; He, W.M. Green Chem. 2020, 22,118.
|
|
(b) Wang, Z.; He, W.M. Chin. J. Org. Chem. 2019, 39,3594. (in Chinese)
|
|
|
( 王峥, 何卫民, 有机化学, 2019, 39,3594.)
|
|
|
(c) Wu, Y.Q.; He, W.M. Chin. J. Org. Chem. 2020, 40,2597. (in Chinese)
|
|
|
( 吴燕, 何卫民, 有机化学, 2020, 40,2597.)
|
|
|
(d) Zhang, P.; Shi, H.N.; Zhang, T.S.; Cai, P.J.; Jiang, B.; Tu, S.J. Chin. J. Org. Chem. 2020, 40,423. (in Chinese)
|
|
|
( 张萍, 石浩楠, 张天舒, 蔡佩君, 姜波, 屠树江, 有机化学, 2020, 40,423.)
|
|
| [19] |
(a) Bandgar, B.P.; Patil, S.A.; Korbad, B.L.; Bandgar, S.B.; Hote, B.S. Aust. J. Chem. 2008, 61,552.
|
|
(b) Matam, S.K. P.; Perumal, M.S. ChemistrySelect 2017, 2,2363.
|
|
|
(c) da Rocha Pissurno, A.P.; da Silva de Laurentiz, R. Synth. Commun. 2017, 47,1874.
|
|
|
(d) Takefumi, M.; Naoki, T.; Takafumi, N.; Yumi, T. Heterocycles 1988, 27,1907.
|
| [1] | 黄净, 杨毅华, 张占辉, 刘守信. 酰胺键的绿色高效构建方法与技术进展[J]. 有机化学, 2024, 44(2): 409-420. |
| [2] | 蒋宜欣, 唐伯孝, 毛海波, 陈雪霞, 俞洋杰, 全翠英, 徐昭阳, 石金慧, 刘益林. 水-聚乙二醇(PEG-200)中烯烃与碘代芳烃绿色可循环无负载偶联反应的研究[J]. 有机化学, 2023, 43(9): 3210-3215. |
| [3] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [4] | 卢凯, 屈浩琦, 陈樨, 秋慧, 郑晶, 马猛涛. 无催化剂、无溶剂条件下炔烃和烯烃与儿茶酚硼烷的硼氢化反应[J]. 有机化学, 2023, 43(6): 2197-2205. |
| [5] | 窦谦, 汪太民, 房丽晶, 翟宏斌, 程斌. 光诱导铁催化在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1386-1415. |
| [6] | 莫百川, 陈春霞, 彭进松. 木质素及其衍生物负载金属催化剂在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1215-1240. |
| [7] | 李奇阳, 张海燕, 刘文博. 无过渡金属参与的碳硅键构筑方法研究进展[J]. 有机化学, 2023, 43(10): 3470-3490. |
| [8] | 宇世伟, 陈兆华, 陈淇, 林舒婷, 何金萍, 陶冠燊, 汪朝阳. 硫代磺酸酯的合成与应用研究进展[J]. 有机化学, 2022, 42(8): 2322-2330. |
| [9] | 顾清云, 程振凤, 曾小宝. 电化学氧化三氟甲基亚磺酸钠与α-羰基二硫缩烯酮的三氟甲基化反应[J]. 有机化学, 2022, 42(5): 1537-1544. |
| [10] | 郑煜, 钱沈城, 徐鹏程, 郑斌南, 黄申林. 电化学氧化芳基端炔的硫氰化磺化反应[J]. 有机化学, 2022, 42(12): 4275-4281. |
| [11] | 应安国, 白林盛, 侯海亮, 许松林, 鲁小彤, 王丽敏. AlCl3@MNPs催化硫杂Michael加成串联反应研究[J]. 有机化学, 2022, 42(11): 3843-3852. |
| [12] | 李红霞, 陈棚, 伍智林, 陆雨函, 彭俊梅, 陈锦杨, 何卫民. 电化学促进的五元芳香杂环与硫氰酸铵氧化交叉脱氢偶联反应[J]. 有机化学, 2022, 42(10): 3398-3404. |
| [13] | 张瑶瑶, 周丽洁, 韩彪, 李维双, 李博解, 朱磊. 壳聚糖负载铜催化剂在有机反应中的应用研究进展[J]. 有机化学, 2022, 42(1): 33-53. |
| [14] | 蒙泽银, 冯承涛, 徐坤. 基于电化学方法构建碳-氮键的最新研究进展[J]. 有机化学, 2021, 41(7): 2535-2570. |
| [15] | 赵志恒, 李鸣, 周娅琴, 何永辉, 张丽珠, 李干鹏, 谷利军. 电化学脱氢[3+2]环化反应合成取代的1,2,4-三氮唑衍生物[J]. 有机化学, 2021, 41(6): 2476-2484. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||