

Selectfluor氧化合成2-芳基苯并噻唑的新方法
收稿日期: 2021-05-07
修回日期: 2021-06-07
网络出版日期: 2021-07-13
基金资助
浙江省领军型创新团队(2019R01005)
A New Method for the Synthesis of 2-Arylbenzothiazoles Oxidized by Selectfluor
Received date: 2021-05-07
Revised date: 2021-06-07
Online published: 2021-07-13
Supported by
Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang(2019R01005)
傅琴姣 , 张瑞芹 , 裘浣沂 , 马仁超 , 马永敏 . Selectfluor氧化合成2-芳基苯并噻唑的新方法[J]. 有机化学, 2021 , 41(9) : 3585 -3592 . DOI: 10.6023/cjoc202105010
2-Arylbenzothiazoles were effectively synthesized via an oxidative process by Selectfluor in 1-butanol at 120 ℃, using 2-arylbenzothiazoles and aryl/aliphatic ketones as starting materials. Bioactive pharmaceutical intermediates were obtained by selecting substituents on the ring of aryl methyl ketones.
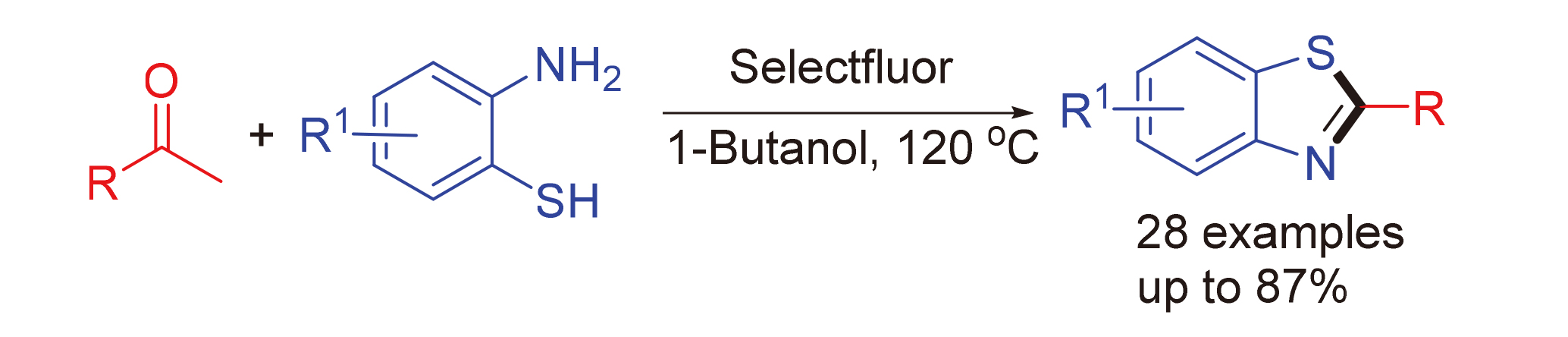
Key words: Selectfluor; 2-arylbenzothiazole; aryl methyl ketone; 2-aminobenzenethiol; oxidation
| [1] | (a) Zablotskaya, A.; Segal, I.; Geronikaki, A.; Eremkina, T.; Belyakov, S.; Petrova, M.; Shestakova, I.; Zvejniece, L.; Nikolajeva, V. Eur. J. Med. Chem. 2013, 70, 846. |
| [1] | (b) Cai, J.; Sun, M.; Wu, X.; Chen, J.; Wang, P.; Zong, X.; Ji, M. Eur. J. Med. Chem. 2013, 63, 702. |
| [1] | (c) Huang, S.-T.; Hsei, I. J.; Chen, C. Bioorg. Med. Chem. 2006, 14, 6106. |
| [2] | (a) Diao, P.-C.; Lin, W.-Y.; Jian, X.-E.; Li, Y.-H.; You, W.-W.; Zhao, P.-L. Eur. J. Med. Chem. 2019, 179, 196. |
| [2] | (b) Racane, L.; Pticek, L.; Fajdetic, G.; Tralic-Kulenovic, V.; Klobucar, M.; Pavelic, S. K.; Peric, M.; Paljetak, H. C.; Verbanac, D.; Starcevic, K. Bioorg. Chem. 2019, 95, 103537. |
| [2] | (c) Li, E.; Meng, Y.; Zhang, L.; Zhang, Y.; Zhou, R.; Liu, L.; Li, N.; Xin, J.; Zheng, J.; Shan, L.; Liu, H.; Zhang, Q. Chin. J. Org. Chem. 2020, 40, 417. (in Chinese). |
| [2] | ( 李二冬, 孟娅琪, 张路野, 张洋, 周蕊, 刘丽敏, 栗娜, 辛景超, 郑甲信, 单丽红, 刘宏民, 张秋荣, 有机化学, 2020, 40, 417.) |
| [3] | (a) Keri, R. S.; Patil, M. R.; Patil, S. A.; Budagumpi, S. Eur. J. Med. Chem. 2015, 89, 207. |
| [3] | (b) Sumit, K. A.; Kumar, A.; Mishra, A. K. Mini-Rev. Med. Chem. 2020, 21, 314. |
| [4] | (a) Huang, X.; Meggers, E. Acc. Chem. Res. 2019, 52, 833. |
| [4] | (b) Lu, H.-Y.; Barve, I. J.; Selvaraju, M.; Sun, C.-M. ACS Comb. Sci. 2020, 22, 42. |
| [4] | (c) Huo, X.; Li, W.; Zhang, B.; Chen, X.; Zhou, Y.; Zhang, J.; Han, X.; Dai, B. Chin. J. Org. Chem. 2018, 38, 3356. (in Chinese). |
| [4] | ( 霍新玉, 李文斌, 张博雅, 陈晓飞, 周月婷, 张洁, 韩小强, 代斌, 有机化学, 2018, 38, 3356.) |
| [4] | (d) Jiang, K.; Cao, L.; Hao, Z.; Chen, M.; Cheng, J.; Li, X.; Xiao, P.; Chen, L.; Wang, Z. Chin. J. Org. Chem. 2017, 37, 2221. (in Chinese). |
| [4] | ( 蒋凯, 曹梁, 郝志峰, 陈美燕, 程洁銮, 李晓, 肖萍, 陈亮, 汪朝阳, 有机化学, 2017, 37, 2221.) |
| [4] | (e) Gabr, M. T.; El-Gohary, N. S.; El-Bendary, E. R.; El-Kerdawy, M. M.; Ni, N. Chin. Chem. Lett. 2016, 27, 380. |
| [5] | (a) Guria, U. N.; Gangopadhyay, A.; Ali, S. S.; Maiti, K.; Samanta, S. K.; Manna, S.; Ghosh, A. K.; Uddin, M. R.; Mandal, S.; Mahapatra, A. K. Anal. Methods 2019, 11, 5447. |
| [5] | (b) Maliyappa, M. R.; Keshavayya, J.; Mahanthappa, M.; Shivaraj, Y.; Basavarajappa, K. V. J. Mol. Struct. 2020, 1199, 126959. |
| [6] | (a) Zhang, F.; Xu, J.; Guo, X.; Yuan, B.; Huang, H.; Li, L. Appl. Surf. Sci. 2020, 501, 144054. |
| [6] | (b) Devi, A. S.; Aswathy, V. V.; Mary, Y. S.; Panicker, C. Y.; Armakovic, S.; Armakovic, S. J.; Ravindran, R.; Van Alsenoy, C. J. Mol. Struct. 2017, 1148, 282. |
| [6] | (c) Dzulkharnien, N. S. F.; Salleh, N. M.; Yahya, R.; Karim, M. R. Soft Mater. 2017, 15, 292. |
| [7] | Wang, Y.; Zhou, R.; Liu, W.; Liu, C.; Wu, P. Chin. Chem. Lett. 2020, 31, 2950. |
| [8] | (a) Zhang, J.; Liu, Y.; Zhang, Y.; Hu, L.; Han, S. Chin. J. Org. Chem. 2021, 41, 1053. (in Chinese). |
| [8] | ( 张俊, 刘雅菲, 张育榕, 呼亮, 韩世清, 有机化学, 2021, 41, 1053.) |
| [8] | (b) Dai, X.; Zhu, Y.; Wang, Z.; Weng, J. Chin. J. Org. Chem. 2017, 37, 1924. (in Chinese). |
| [8] | ( 戴小强, 朱亚波, 汪洲洋, 翁建全, 有机化学, 2017, 37, 1924.) |
| [8] | (c) Zhu, N.; Zhang, Z.; Gao, M.; Han, L.; Suo, Q.; Hong, H. Chin. J. Org. Chem. 2013, 33, 1423. (in Chinese). |
| [8] | ( 竺宁, 张志伟, 高敏, 韩利民, 索全伶, 洪海龙, 有机化学, 2013, 33, 1423.) |
| [8] | (d) Yang, W.; Li, B.; Zhang, M.; Wang, S.; Ji, Y.; Dong, S. Chin. Chem. Lett. 2020, 31, 1313. |
| [8] | (e) Wan, J.-P.; Zhou, Y.; Liu, Y.; Sheng, S. Green Chem. 2016, 18, 402. |
| [8] | (f) Singh, M.; Vaishali, Paul, A. K.; Singh, V. Org. Biomol. Chem. 2020, 18, 4459. |
| [9] | (a) Ferlin, F.; van der Hulst, M. K.; Santoro, S.; Lanari, D.; Vaccaro, L. Green Chem. 2019, 21, 5298. |
| [9] | (b) Fan, L.-Y.; Shang, Y.-H.; Li, X.-X.; Hua, W.-J. Chin. Chem. Lett. 2015, 26, 77. |
| [9] | (c) Samanta, S.; Das, S.; Biswas, P. J. Org. Chem. 2013, 78, 11184. |
| [9] | (d) Mali, J. K.; Mali, D. A.; Telvekar, V. N. Tetrahedron. Lett. 2016, 57, 2324. |
| [9] | (e) Nagao, I.; Ishizaka, T.; Kawanami, H. Green Chem. 2016, 18, 3494. |
| [9] | (f) Sun, Y.; Jiang, H.; Wu, W.; Zeng, W.; Wu, X. Org. Lett. 2013, 15, 1598. |
| [9] | (g) Ma, R.; Ding, Y.; Chen, R.; Wang, Z.; Wang, L.; Ma, Y. J. Org. Chem. 2021, 86, 310. |
| [9] | (h) Sharma, H.; Singh, N.; Jang, D. O, Green Chem. 2014, 16, 4922. |
| [9] | (i) Li, Z.; Dong, J.; Chen, X.; Li, Q.; Zhou, Y.; Yin, S.-F. J. Org. Chem. 2015, 80, 9392. |
| [9] | (j) Liu, J.; Wang, C.; Ma, X.; Shi, X.; Wang, X.; Li, H.; Xu, Q. Catal. Lett. 2016, 146, 2139. |
| [9] | (k) Shi, X.; Guo, J. Liu, J.; Ye, M.; Xu, Q. Chem.-Eur. J. 2015, 21, 9988. |
| [10] | (a) Xu, Y.; Li, B.; Zhang, X.; Fan, X. J. Org. Chem. 2017, 82, 9637. |
| [10] | (b) Mishra, N.; Singh, A. S.; Agrahari, A. K.; Singh, S. K.; Singh, M.; Tiwari, V. K. ACS Comb. Sci. 2019, 21, 389. |
| [10] | (c) Urzúa, J. I.; Contreras, R.; Salas, C. O.; Tapia, R. A. RSC Adv. 2016, 6, 82401. |
| [11] | Ma, D.; Xie, S.; Xue, P.; Zhang, X.; Dong, J.; Jiang, Y. Angew. Chem., Int. Ed. 2009, 48, 4222. |
| [12] | (a) Zhu, Y.-P.; Jia, F.-C.; Liu, M.-C.; Wu, A.-X. Org. Lett. 2012, 14, 4414. |
| [12] | (b) Zhu, Y.-P.; Lian, M.; Jia, F.-C.; Liu, M.-C.; Yuan, J.-J.; Gao, Q.-H.; Wu, A.-X. Chem. Commun. 2012, 48, 9086. |
| [13] | (a) Liao, Y.; Qi, H.; Chen, S.; Jiang, P.; Zhou, W.; Deng, G.-J. Org. Lett. 2012, 14, 6004. |
| [13] | (b) Wang, J.; Zhang, X.-Z.; Chen, S.-Y.; Yu, X.-Q. Tetrahedron 2014, 70, 245. |
| [13] | (c) Liu, S.; Chen, R.; Chen, H.; Deng, G.-J. Tetrahedron Lett. 2013, 54, 3838. |
| [14] | (a) Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Chem. Rev. 2021, 121, 506. |
| [14] | (b) Wan, J.-P.; Gao, Y.; Wei, L. Chem.-Asian J. 2016, 11, 2092. |
| [14] | Gan, L.; Yu, Q.; Liu, Y.; Wan, J.-P. J. Org. Chem. 2021, 86, 1231. |
| [14] | (d) Gan, Y.; Zhang, N.; Huang, S.; Liu, Y. Chin. J. Chem. 2020, 38, 1686. |
| [14] | (e) Wang, G.; Guo, Y.; Wan, J. Chin. J. Org. Chem. 2020, 40, 645. (in Chinese). |
| [14] | ( 王国栋, 郭艳辉, 万结平, 有机化学, 2020, 40, 645.) |
| [15] | (a) Nyffeler, P. T.; Duron, S. G.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem.,Int. Ed. 2004, 44, 192. |
| [15] | (b) Syvret, R. G.; Butt, K. M.; Nguyen, T. P.; Bulleck, V. L.; Rieth, R. D. J. Org. Chem. 2002, 67, 4487. |
| [16] | Lv, Y.; Sun, K.; Pu, W.; Mao, S.; Li, G.; Niu, J.; Chen, Q.; Wang, T. RSC Adv. 2016, 6, 93486. |
| [17] | Zhao, J.; Xiao, Q.; Chen, J.; Xu, J. Eur. J. Org. Chem. 2020, 2020, 5201. |
| [18] | Wang, H.; Ren, S.; Zhang, J.; Zhang, W.; Liu, Y. J. Org. Chem. 2015, 80, 6856. |
| [19] | (a) Shah, S.; Arshia; Javaid, K.; Zafar, H.; Khan, K. M.; Khalil, R.; Ul-Haq, Z.; Perveen, S.; Choudhary, M. I. Bioorg. Chem. 2018, 78, 269. |
| [19] | (b) Ghannam, I. A. Y.; Abd El-Meguid, E. A.; Ali, I. H.; Sheir, D. H.; El Kerdawy, A. M. Bioorg. Chem. 2019, 93, 103373. |
| [20] | (a) Deligeorgiev, T. G.; Kaloyanova, S.; Vasilev, A.; Vaquero, J. J., Phosphorus, Sulfur Silicon Relat. Elem. 2010, 185, 2292. |
| [20] | (b) Fawzia, A. Q.; Ramadan, A. M.; Kamal, U. S. Molecules 2008, 13, 2908. |
| [20] | (c) Kiyofumi, I.; Chisa, H.; Kou, H.; Takayuki, D. Org. Lett. 2008, 10, 5147. |
| [20] | (d) Wang, Z.; Tang, R.; Li, J. Chin. J. Chem. 2011, 29, 314. |
| [20] | (e) Das, K.; Mondal, A.; Srimani, D. Chem. Commun. 2018, 54, 10582. |
| [20] | (f) Xu, Z.-M.; Li, H.-X.; Young, D. J.; Zhu, D.-L.; Li, H.-Y.; Lang, J.-P. Org. Lett. 2019, 21, 237. |
| [20] | (g) Song, Q.; Feng, Q.; Zhou, M. Org. Lett. 2013, 15, 5990. |
/
| 〈 |
|
〉 |