

过渡金属催化的C—P键活化
收稿日期: 2021-05-31
修回日期: 2021-07-12
网络出版日期: 2021-07-26
基金资助
国家自然科学基金(21871145); 国家自然科学基金(91856104); 天津市自然科学基金(19JCZDJC37900)
Transition Metal-Catalyzed C—P Bond Activation
Received date: 2021-05-31
Revised date: 2021-07-12
Online published: 2021-07-26
Supported by
National Natural Science Foundation of China(21871145); National Natural Science Foundation of China(91856104); Natural Science Foundation of Tianjin City(19JCZDJC37900)
张凤萍 , 栾玉新 , 叶萌春 . 过渡金属催化的C—P键活化[J]. 有机化学, 2021 , 41(10) : 3880 -3891 . DOI: 10.6023/cjoc202105053
Transition metal-catalyzed C—P bond activation provides an ecomomical and high-efficient route for the synthesis of organic phosphine compounds, and has received increasing attention in recent years. Owing to high bond energy of C—P bond and strong coordinative ability of P atom, the activation of C—P bond has been a fomidable challenge. Relying on substrate-preactivation and various catalysts, great progress has been achieved. This review will give a summary of this field, and according to different mechanisms of C—P bond activation and strategies of substrate pre-activation, a detailed description on reaction type, development, characteristics and mechanism will be made.
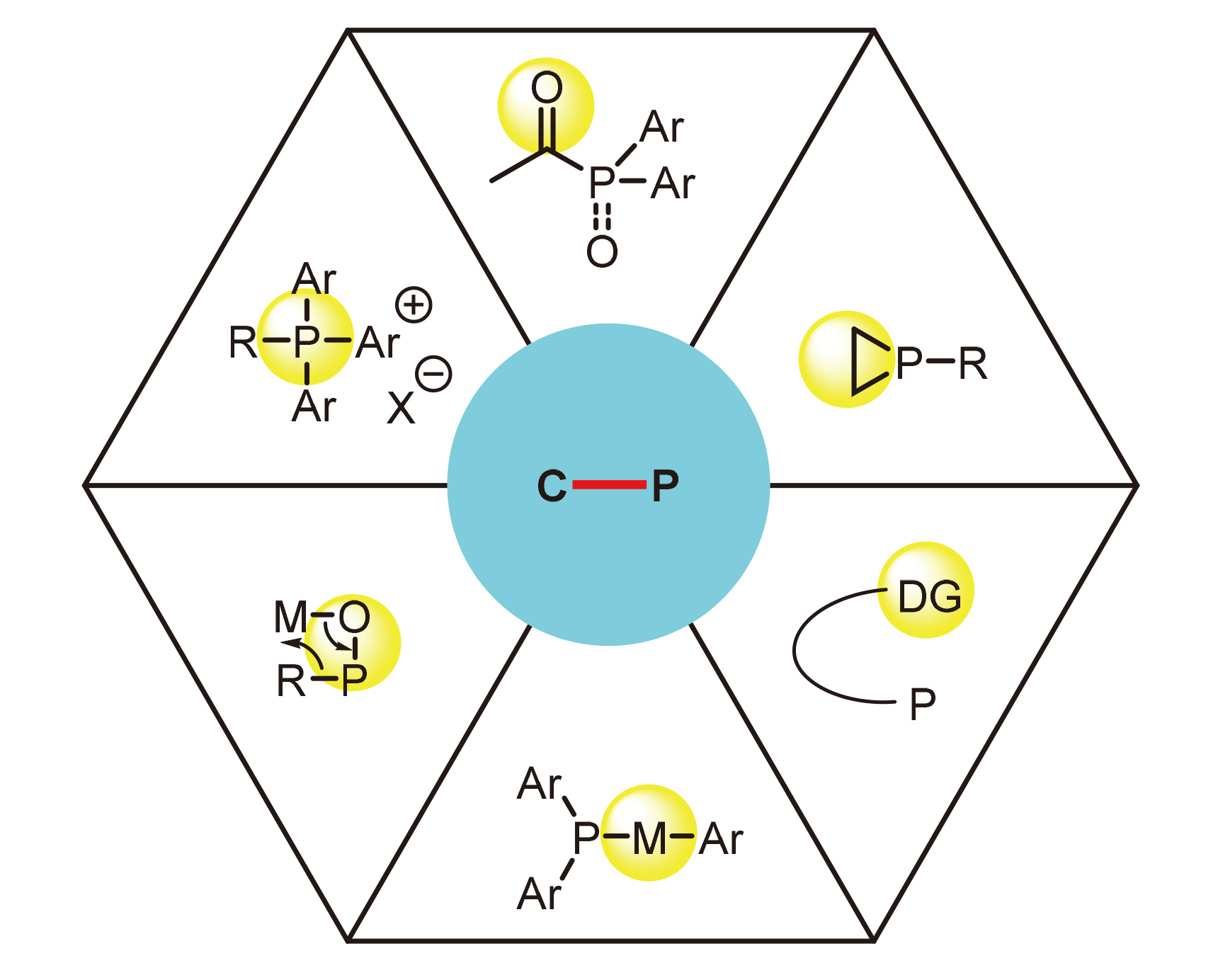
Key words: organophosphines; transition metal; C—P bond activation
| [1] | (a) Fields, S. C. Tetrahedron 1999, 55, 12237. |
| [1] | (b) Engel, R. Chem. Rev. 1977, 77, 349. |
| [2] | (a) Wittig, G.; Geissler, G. Justus Liebigs Ann. Chem. 1953, 580, 44. |
| [2] | (b) Lu, X. Y.; Zhang, C. M.; Xu, Z. R. Acc. Chem. Res. 2001, 34, 535. |
| [3] | (a) Trofimov, B. A.; Arbuzova, S. N.; Gusarova, N. K. Russ. Chem. Rev. 1999, 68, 215. |
| [3] | (b) Honaker, M. T.; Hovland, J. M.; Salvatore, R. N. Curr. Org. Chem. 2007, 4, 31. |
| [3] | (c) Schwan, A. L. Chem. Soc. Rev. 2004, 33, 218. |
| [4] | (a) Jiang, T.; Zhang, H.; Ding, Y.; Zou, S.; Chang, R.; Huang, H. Chem. Soc. Rev. 2020, 49, 1487. |
| [4] | (b) Lee, Y. H.; Morandi, B. Coord. Chem. Rev. 2019, 386, 96. |
| [4] | (c) Wang, L.; Chen, H.; Duan, Z. Chem. Asian J. 2018, 13, 2164. |
| [4] | (d) Tappe, F. M. J.; Trepohl, V. T.; Oestreich, M. Synthesis 2010, 3037. |
| [4] | (e) Macgregor, S. A. Chem. Soc. Rev. 2007, 36, 67. |
| [5] | Wei, K.; Luo, K.; Liu, F.; Wu, L.; Wu, L.-Z. Org. Lett. 2019, 21, 1994. |
| [6] | (a) Liu, T.; Zhu, J.; Sun, X.; Cheng, L.; Wu, L. Adv. Synth. Catal. 2019, 361, 3532. |
| [6] | (b) Hou, F.; Du, X.-P.; Alduma, A. I.; Li, Z.-F.; Huo, C.-D. Wang, X.-C.; Wu, X.-F.; Quan, Z.-J. Adv. Synth. Catal. 2020, 362, 4755. |
| [6] | (c) Song, Y.; Wang, L.; Duan, Z.; Mathey, F. Chin. Chem. Lett. 2020, 31, 329. |
| [7] | For selected reviews on C—H activation, see: (a) Gandeepan, P.; Mu?ller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. Chem. Rev. 2019, 119, 2192. |
| [7] | (b) Zhang, Q.; Shi, B. F. Acc. Chem. Res. 2021, 54, 2750. |
| [7] | (c) He, J.; Wasa, M.; Chan, Kelvin S. L.; Shao, Q.; Yu, J. Q. Chem. Rev. 2017, 117, 8754. |
| [7] | (d) Davison, R. T.; Kuker, E. L.; Dong, V. M. Acc. Chem. Res. 2021, 54, 1067. |
| [7] | (e) Ye, B.; Cramer, N. Acc. Chem. Res. 2015, 48, 1308. |
| [7] | (f) Hummel, J. R.; Boerth, J. A.; Ellman, J. A. Chem. Rev. 2017, 117, 9163. |
| [7] | (g) Dong, Z.; Ren, Z.; Thompson, S.; Xu, Y.; Dong, G. B. Chem. Rev. 2017, 117, 9333. |
| [7] | (h) Hu, Y.; Wang, C. Acta Phys.-Chim. Sin. 2019, 35, 913. |
| [7] | (i) Wang, Y.-X.; Ye, M. Sci. China Chem. 2018, 61, 1004. |
| [7] | (j) Wang, R.; Luan, Y.; Ye, M. Chin. J. Chem. 2019, 37, 720. |
| [7] | (k) Li, R.; Xu, X.; Ye, M. Chin. J. Org. Chem. 2020, 40, 3196. (in Chinese) |
| [7] | (李然, 徐学涛, 叶萌春, 有机化学, 2020, 40, 3196.) |
| [7] | (l) Zhao, M.; Lu, W. Acta Phys.-Chim. Sin. 2019, 35, 977. |
| [8] | For selected reviews on C—C activation, see: (a) Xia, Y.; Dong, G. Nat. Rev. Chem. 2020, 4, 600. |
| [8] | (b) Souillart, L.; Cramer, N. Chem. Rev. 2015, 115, 9410. |
| [8] | (c) Song, F. J.; Gou, T.; Wang, B. Q.; Shi, Z. J. Chem. Soc. Rev. 2018, 47, 7078. |
| [8] | (d) Murakami, M.; Ishida, N. Chem. Rev. 2021, 121, 264. |
| [8] | (e) Wang, W.; Xie, J. Chin. J. Org. Chem. 2020, 40, 1396. (in Chinese) |
| [8] | (王文亮, 谢劲, 有机化学, 2020, 40, 1396.) |
| [9] | For selected reviews on C—O activation, see: (a) Liu, F.; Jiang, H. J.; Zhou, Y.; Shi, Z. J. Chin. J. Chem. 2020, 38, 855. |
| [9] | (b) Tobisu, M.; Chatani, N. Acc. Chem. Res. 2015, 48, 1717. |
| [9] | (c) Su, B.; Cao, Z.-C.; Shi, Z.-J. Acc. Chem. Res. 2015, 48, 886. |
| [9] | (d) Goossen, L. J.; Goossen, K.; Stanciu, C. Angew. Chem., Int. Ed. 2009, 48, 3569. |
| [10] | For selected reviews on C—N activation, see: (a) García-Cárceles, J.; Bahou, K. A.; Bower, J. F. ACS Catal. 2020, 10, 12738. |
| [10] | (b) Wang, Q. J.; Su, Y. J.; Li, L. X.; Huang, H. M. Chem. Soc. Rev. 2016, 45, 1257. |
| [10] | (c) Boit, T. B.; Bulger, A. S.; Dander, J. E.; Garg, N. K. ACS Catal. 2020, 10, 12109. |
| [10] | (d) Kaiser, D.; Bauer, A.; Lemmerer, M.; Maulide, N. Chem. Soc. Rev. 2018, 47, 7899. |
| [10] | (e) Gao, Y.; Ji, C.-L.; Hong, X. Sci. China Chem. 2017, 60, 1413. |
| [11] | O'Keefe, D. F.; Dannock, M. C.; Marcuccio, S. M. Tetrahedron Lett. 1992, 33, 6679. |
| [12] | Sakamoto, M.; Shimizu, I.; Yamamoto, A. Chem. Lett. 1995, 24, 1101. |
| [13] | Brunker, T. J.; Moncarz, J. R.; Glueck, D. S.; Zakharov, L. N.; Golen, J. A.; Rheingold, A. L. Organometallics 2004, 23, 2228. |
| [14] | Marinetti, A.; Carmichael, D. Chem. Rev. 2002, 102, 201. |
| [15] | Landis, C. R.; Nelson, R. C; Jin, W.; Bowman, A. C. Organometallics 2006, 25, 1377. |
| [16] | Ru?nzi, T.; Tritschler, U.; Roesle, P.; Go?ttker-Schnetmann, I.; Mo?ller, H. M.; Caporaso, L.; Poater, A.; Cavallo, L.; Mecking, S. Organometallics 2012, 31, 8388. |
| [17] | Alcazar-Roman, L. M.; Hartwig, J. F.; Rheingold, A. L.; Liable-Sands, L. M.; Guzei, I. A. J. Am. Chem. Soc. 2000, 122, 4618. |
| [18] | Ge, S. Z.; Green, R. A.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 1617. |
| [19] | Ai, P. F.; Danopoulos, A. A.; Braunstein, P. Dalton Trans. 2014, 43, 1957. |
| [20] | Qin, H.-L.; Leng, J.; Zhang, W.; Kantchev, E. A. B. Dalton Trans. 2018, 47, 2662. |
| [21] | Vicente, J.; Abad, J.-A.; López-Nicolás, R.-M.; Jones, P. G. Organometallics 2004, 23, 4325. |
| [22] | Hwang, L. K.; Na, Y.; Lee, J.; Do, Y.; Chang, S. Angew. Chem., Int. Ed. 2005, 44, 6166. |
| [23] | Zhang, X.; McNally, A. Angew. Chem., Int. Ed. 2017, 56, 9833. |
| [24] | Segelstein, B. E.; Butler, T. W.; Chenard, B. L. J. Org. Chem. 1995, 60, 12. |
| [25] | Kong, K. C.; Cheng, C. H. J. Am. Chem. Soc. 1991, 113, 6313. |
| [26] | Goodson, F. E.; Wallow, T. I.; Novak, B. M. J. Am. Chem. Soc. 1997, 119,12441. |
| [27] | (a) Kwong, F. Y.; Chan, K. S. Chem. Commun. 2000, 1069. |
| [27] | (b) Kwong, F. Y.; Lai, C. W.; Tian, Y.; Chan, K. S. Tetrahedron Lett. 2000, 41,10285. |
| [27] | (c) Kwong, F. Y.; Lai, C. W.; Yu, M.; Chan, K. S. Tetrahedron 2004, 60, 5635. |
| [28] | Baba, K.; Tobisu, M.; Chatani, N. Angew. Chem., Int. Ed. 2013, 52, 11892. |
| [29] | Baba, K.; Tobisu, M.; Chatani, N. Org. Lett. 2015, 17, 70. |
| [30] | Zhou, H.; Li, J.; Yang, H.; Xia, C.; Jiang, G. Org. Lett. 2015, 17, 4628. |
| [31] | Yu, R.; Chen, X.; Wang, Z. Tetrahedron Lett. 2016, 57, 3404. |
| [32] | Lian, Z.; Bhawal, B. N.; Yu, P.; Morandi, B. Science 2017, 356, 1059. |
| [33] | Cao, J.; Huang, X.; Wu, L. Chem. Commun. 2013, 49, 7747. |
| [34] | For nickel intermediates with negative charge, see: (a) Gartia, Y.; Ramidi, P.; Jones, D. E.; Pulla, S.; Ghosh, A. Catal. Lett. 2014, 144, 507. |
| [34] | (b) Sahoo, D.; Yoo, C.; Lee, Y. J. Am. Chem. Soc. 2018, 140, 2179. |
| [35] | Zhou, Y.; Gan, Z. J.; Su, B.; Li, J.; Duan, Z.; Mathey, F. Org. Lett. 2015, 17, 5722. |
| [36] | Zhu, J.; Mao, M.; Ji, H.-J.; Xu, J.-Y.; Wu, L. Org. Lett. 2017, 19, 1946. |
| [37] | For the coordination of phosphine oxide with metals, see: (a) Veith, M.; Huch, V. J. Organomet. Chem. 1985, 293, 161. |
| [37] | (b) Zabula, A. V.; Pape, T.; Hepp, A.; Hahn, F. E. Dalton Trans. 2008, 5886. |
| [38] | Nakazawa, H.; Matsuoka, Y.; Yamaguchi, H.; Kuroiwa, T.; Miyoshi, K.; Yoneda, H. Organometallics 1989, 8, 2272. |
| [39] | Yu, R.; Chen, X.; Martin, S. F.; Wang, Z. Org. Lett. 2017, 19, 1808. |
| [40] | Chen, X.; Liu, X.; Zhu, H.; Wang, Z. Tetrahedron 2021, 81, 131912. |
| [41] | Liedtke, J.; Rüegger, H.; Loss, S.; Grützmacher, H. Angew. Chem., Int. Ed. 2000, 39, 2478. |
| [42] | Heyn, R. H.; Görbitz, C. H. Organometallics 2002, 21, 2781. |
| [43] | Derrah, E. J.; Ladeira, S.; Bouhadir, G.; Miqueu, K.; Bourissou, D. Chem. Commun. 2011, 47, 8611. |
| [44] | Abatjoglou, A. G.; Bryant, D. R. Organometallics 1984, 3, 932. |
| [45] | Sabater, S.; Page, M. J.; Mahon, M. F.; Whittlesey, M. K. Organometallics 2017, 36, 1776. |
| [46] | Inoue, A.; Shinokubo, H.; Oshima, K. J. Am. Chem. Soc. 2003, 125, 1484. |
| [47] | Masuda, K.; Sakiyama, N.; Tanaka, R.; Noguchi, K.; Tanaka, K. J. Am. Chem. Soc. 2011, 133, 6918. |
/
| 〈 |
|
〉 |