

铁(0)促进的醛与溴代硝基甲烷的Henry型反应高效合成2-硝基-1-醇
收稿日期: 2021-07-23
修回日期: 2021-08-27
网络出版日期: 2021-08-29
基金资助
南京工业大学科研启动基金(39837118); 南京工业大学科研启动基金(39837146); 国家自然科学基金(22001121); 江苏省自然科学基金(BK20180690)
Iron(0)-Mediated Henry-Type Reaction of Bromonitromethane with Aldehydes for the Efficient Synthesis of 2-Nitro-alkan-1-ols
Received date: 2021-07-23
Revised date: 2021-08-27
Online published: 2021-08-29
Supported by
Nanjing Tech University(39837146); National Natural Science Foundation of China(22001121); Natural Science Foundation of Jiangsu Province(BK20180690)
张斯旋 , 李祥瑞 , 李文欣 , 饶卫东 , 葛丹华 , 沈志良 , 褚雪强 . 铁(0)促进的醛与溴代硝基甲烷的Henry型反应高效合成2-硝基-1-醇[J]. 有机化学, 2022 , 42(1) : 235 -241 . DOI: 10.6023/cjoc202107048
The Henry-type reaction of bromonitromethane with various aldehydes for the efficient synthesis of 2-nitro-alkan- 1-ols by using inexpensive and commercial iron powder as reaction mediator was developed. The reaction proceeded efficiently in the presence of PbCl2 and tetrabutylammonium hydrogen sulfate (TBAHS) to produce the desired products in moderate to good yield with wide functional group tolerance.
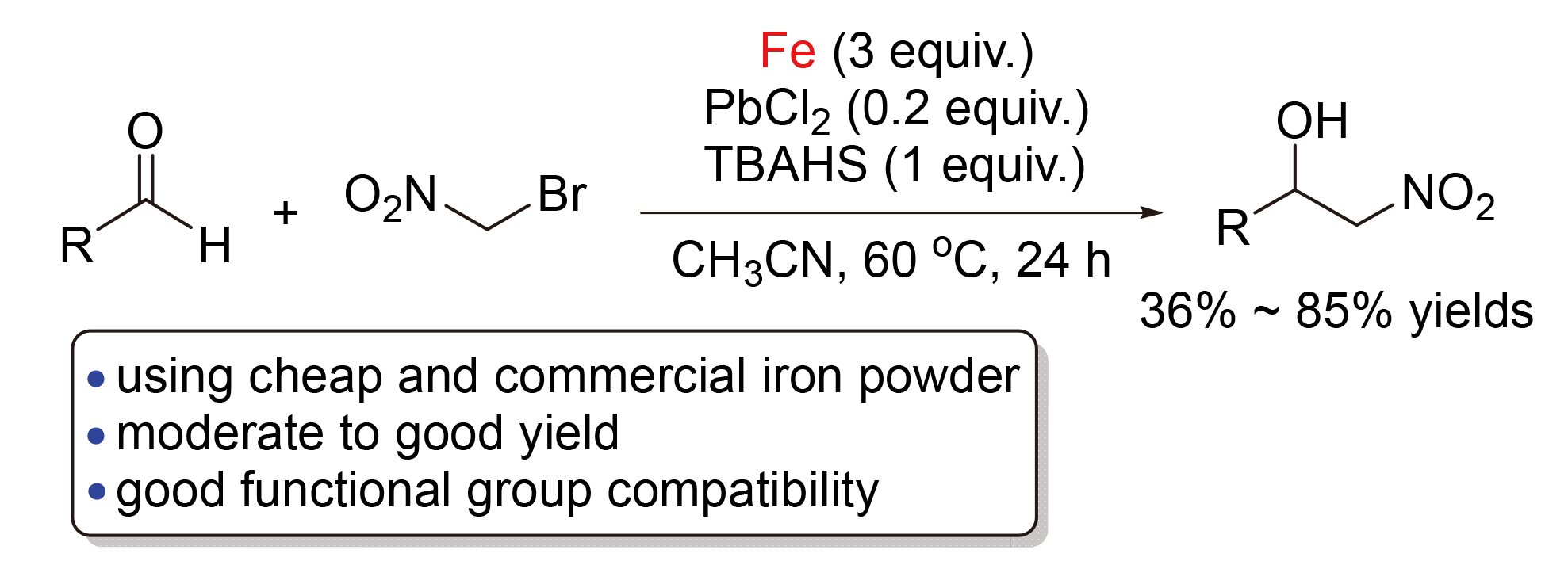
Key words: iron; lead dichloride; Henry-type reaction; bromonitromethane; 2-nitro-alkan-1-ol
| [1] | (a) Ono, N. In The Nitro Group in Organic Synthesis, Wiley-VCH, New York, 2001, p. 30. |
| [1] | (b) Norman, B. H.; Morris, M. L. Tetrahedron Lett. 1992, 33, 6803. |
| [1] | (c) Kawabata, T.; Kiryu, Y.; Sugiure, Y.; Fuji, K. Tetrahedron Lett. 1993, 34, 5127. |
| [1] | (d) Sasai, H.; Kim, W.-S.; Suzuki, T.; Shibasaki, M. Tetrahedron Lett. 1994, 35, 6123. |
| [1] | (e) Corey, E. J.; Zhang, F.-Y. Angew. Chem. Int. Ed. 1999, 38, 1931. |
| [1] | (f) Grembecka, J.; Kafarski, P. Mini Rev. Med. Chem. 2001, 1, 133. |
| [2] | Henry, L. Bull. Soc. Chim. Fr. 1895, 13, 999. |
| [3] | (a) Rosini, G.; Ballini, R. Synthesis 1988, 833. |
| [3] | (b) Shvekhgeimer, M. A. Russ. Chem. Rev. 1998, 67, 35. |
| [3] | (c) Luzzio, F. A. Tetrahedron 2001, 57, 915. |
| [4] | (a) Concellon, J. M.; Rodriguez-Solla, H.; Concellon, C.; Garcia-Granda, S.; Diaz, M. R. Org. Lett. 2006, 8, 5979. |
| [4] | (b) Alcaide, B.; Almendros, P.; Luna, A.; Torres, M. R. Org. Biomol. Chem. 2008, 6, 1635. |
| [4] | (c) Blay, G.; Hernandez-Olmos, V.; Pedro, J. R. Chem. Commun. 2008, 4840. |
| [4] | (d) Leighty, M. W.; Shen, B.; Johnston, J. N. J. Am. Chem. Soc. 2012, 134, 15233. |
| [4] | (e) Mao, P.; Yang, L.; Xiao, Y.; Yuan, J.; Mai, W.; Gao, J.; Zhang, X. Chin. J. Org. Chem. 2019, 39, 443. |
| [4] | (f) Hu, Z.; Jiang, G.; Zhu, Z. Gong, B.; Xie, Z.; Le, Z. Chin. J. Org. Chem. 2021, 41, 325. |
| [5] | Concellon, J. M.; Rodriguez-Solla, H.; Concellon, C. J. Org. Chem. 2006, 71, 7919. |
| [6] | (a) Soengas, R. G.; Estévez, A. M. Eur. J. Org. Chem. 2010, 5190. |
| [6] | (b) Soengas, R. G.; Estévez, A. M. Synlett 2010, 2625. |
| [6] | (c) Soengas, R. G.; Estévez, A. M. Tetrahedron Lett. 2012, 53, 570. |
| [7] | Soengas, R. G.; Silva, A. M. S. Synlett 2012, 873. |
| [8] | Mahasneh, A. S. Z. Naturforsch. 2005, 60b, 416. |
| [9] | (a) Liu, Y.; Lu, Y.; Prashad, M.; Repic, O.; Blacklock, T. J. Adv. Synth. Catal. 2005, 347, 217. |
| [9] | (b) Gao, G.; Tao, Y.; Jiang, J. Green Chem. 2008, 10, 439. |
| [9] | (c) Dey, R.; Mukherjee, N.; Ahammed, S.; Ranu, B. C. Chem. Commun. 2012, 48, 7982. |
| [9] | (d) Liu, X.-Y.; Cheng, B.-Q.; Guo, Y.-C.; Chu, X.-Q.; Rao, W.; Loh, T.-P.; Shen, Z.-L. Org. Chem. Front. 2019, 6, 1581. |
| [9] | (e) Liu, X.-Y.; Li, X.-R.; Zhang, C.; Chu, X.-Q.; Rao, W.; Loh, T.-P.; Shen, Z.-L. Org. Lett. 2019, 21, 5873. |
| [9] | (f) Chan, T. C.; Lau, C. P.; Chan, T. H. Tetrahedron Lett. 2004, 45, 4189. |
| [10] | (a) Blümke, T. D.; Chen, Y.-H.; Peng, Z.; Knochel, P. Nat. Chem. 2010, 2, 313. |
| [10] | (b) Chen, B.-Z.; Wang, C.-X.; Jing, Z.-H.; Chu, X.-Q.; Loh, T.-P.; Shen, Z.-L. Org. Chem. Front. 2019, 6, 313. |
| [11] | (a) Blümke, T. D.; Klatt, T.; Koszinowski, K.; Knochel, P. Angew. Chem. Int. Ed. 2012, 51, 9926. |
| [11] | (b) Takai, K.; Ikawa, Y. Org. Lett. 2002, 4, 1727. |
| [11] | (c) Yun, J.-J.; Zhi, M.-L.; Shi, W.-X.; Chu, X.-Q.; Shen, Z.-L.; Loh, T.-P. Adv. Synth. Catal. 2018, 360, 2632. |
| [11] | (d) Shen, L.; Zhao, K.; Doitomi, K.; Ganguly, R.; Li, Y.-X.; Shen, Z.-L.; Hirao, H.; Loh, T.-P. J. Am. Chem. Soc. 2017, 139, 13570. |
| [12] | (a) Ollevier, T. Org. Biomol. Chem. 2013, 11, 2740. |
| [12] | (b) Wu, Z.; Feng, X.-X.; Wang, Q.-D.; Yun, J.-J.; Rao, W.; Yang, J.-M.; Shen, Z.-L. Chin. Chem. Lett. 2020, 31, 1297. |
| [13] | (a) Chen, C.; Liu, P.; Luo, M.; Zeng, X. ACS Catal. 2018, 8, 5864. |
| [13] | (b) Li, Y.; Deng, G.; Zeng, X. Organometallics 2016, 35, 747. |
| [13] | (c) Yun, J.-J.; Liu, X.-Y.; Deng, W.; Chu, X.-Q.; Shen, Z.-L.; Loh, T.-P. J. Org. Chem. 2018, 83, 10898. |
| [14] | (a) Enthaler, S.; Junge, K.; Beller, M. Angew. Chem. Int. Ed. 2008, 47, 3317. |
| [14] | (b) Bauer, I.; Knölker, H.-J. Chem. Rev. 2015, 115, 3170. |
| [14] | (c) Xiong, H.; Ramkumar, N.; Chiou, M.-F.; Jian, W.; Li, Y.; Su, J.-H.; Zhang, X.; Bao, H. Nat. Commun. 2019, 10, 122. |
| [14] | (d) Wei, R.; Xiong, H.; Ye, C.; Li, Y.; Bao, H. Org. Lett. 2020, 22, 3195. |
| [14] | (e) Deng, W.; Ye, C.; Li, Y.; Li, D.; Bao, H. Org. Lett. 2019, 21, 261. |
| [14] | (f) He, Z.; Fan, M.; Xu, J.; Hu, Y.; Wang, L.; Wu, X.; Xia, C.; Liu, C. Chin. J. Org. Chem. 2019, 39, 3438. |
| [15] | (a) Takai, K.; Kakiuchi, T.; Utimoto, K. J. Org. Chem. 1994, 59, 2671. |
| [15] | (b) Cheng, B.-Q.; Zhao, S.-W.; Song, X.-D.; Chu, X.-Q.; Rao, W.; Loh, T.-P.; Shen, Z.-L. J. Org. Chem. 2019, 84, 5348. |
| [16] | (a) Cheng, B.-Q.; Zhang, S.-X.; Cui, Y.-Y.; Chu, X.-Q.; Rao, W.; Xu, H.; Han, G.-Z.; Shen, Z.-L. Org. Lett. 2020, 22, 5456. |
| [16] | (b) Chu, X.-Q.; Cheng, B.-Q.; Zhang, Y.-W.; Ge, D.; Shen, Z.-L.; Loh, T.-P. Chem. Commun. 2018, 54, 2615. |
| [16] | (c) Wang, P.; Chen, B.-Z.; Guo, Y.-C.; Rao, W.; Shen, Z.-L. Tetrahedron Lett. 2019, 60, 151288. |
| [16] | (d) Wu, L.-H.; Zhao, K.; Shen, Z.-L.; Loh, T.-P. Org. Chem. Front. 2017, 4, 1872. |
| [16] | (e) Liu, B.; Xu, X.; Huang, L.; Feng, H. Chin. J. Org. Chem. 2020, 40, 1290. |
| [16] | (f) Huang, S.; Nie, Y.; Yang, J.; Zheng, Z.; Cao, J.; Xu, Z.; Xu, L. Chin. J. Org. Chem. 2020, 40, 2018. |
| [17] | Wu, Z.; Feng, X.-X.; Wang, Q.-D.; Liu, X.-Y.; Rao, W.; Yang, J.-M.; Shen, Z.-L. Chin. Chem. Lett. 2020, 31, 391. |
| [18] | (a) Chen, B.-Z.; Zhi, M.-L.; Wang, C.-X.; Chu, X.-Q.; Shen, Z.-L.; Loh, T.-P. Org. Lett. 2018, 20, 1902. |
| [18] | (b) Liu, X.-Y.; Cheng, B.-Q.; Guo, Y.-C.; Chu, X.-Q.; Li, Y.-X.; Loh, T.-P.; Shen, Z.-L. Adv. Synth. Catal. 2019, 361, 542. |
| [19] | (a) Tay, N. E. S.; Chen, W.; Levens, A.; Pistritto, V. A.; Huang, Z.; Wu, Z.; Li, Z.; Nicewicz, D. A. Nat. Catal. 2020, 3, 734. |
| [19] | (b) Bhattacharyya, A.; Kavitha, C. V.; Ghorai, M. K.; J. Org. Chem. 2016, 81, 643. |
| [19] | (c) Kirihara, M.; Osugi, R.; Saito, K.; Adachi, K.; Yamazaki, K.; Matsushima, R.; Kimura, Y. J. Org. Chem. 2019, 84, 8330. |
/
| 〈 |
|
〉 |