

对甲苯硫酚/三环己基膦介导的硝基芳烃的光化学还原反应
收稿日期: 2021-09-24
修回日期: 2021-10-26
网络出版日期: 2021-11-03
基金资助
国家自然科学基金(21801051); 国家自然科学基金(21961006); 国家自然科学基金(32172459); 贵州省科技技术基金(黔科合基础-ZK[2021]重点033)
Photochemical Reduction of Nitroaromatics Mediated by p-Toluenethiol/PCy3
Received date: 2021-09-24
Revised date: 2021-10-26
Online published: 2021-11-03
Supported by
National Natural Science Foundation of China(21801051); National Natural Science Foundation of China(21961006); National Natural Science Foundation of China(32172459); Science and Technology Department of Guizhou Province(黔科合基础-ZK[2021]重点033)
鲍兆伟 , 吕洁 , 金智超 . 对甲苯硫酚/三环己基膦介导的硝基芳烃的光化学还原反应[J]. 有机化学, 2021 , 41(12) : 4773 -4779 . DOI: 10.6023/cjoc202109037
A new photochemical reduction of nitroaromatics mediated by p-toluenethiol and PCy3 under solvent-free, metal-free, and mild conditions is reported for the first time. This reaction has shown good generalities, and amine derivatives with different substituents and substitution patterns can be obtained in good yields. Therefore, this reduction method represents a potentially valuable new strategy for the efficient synthesis of amine compounds.
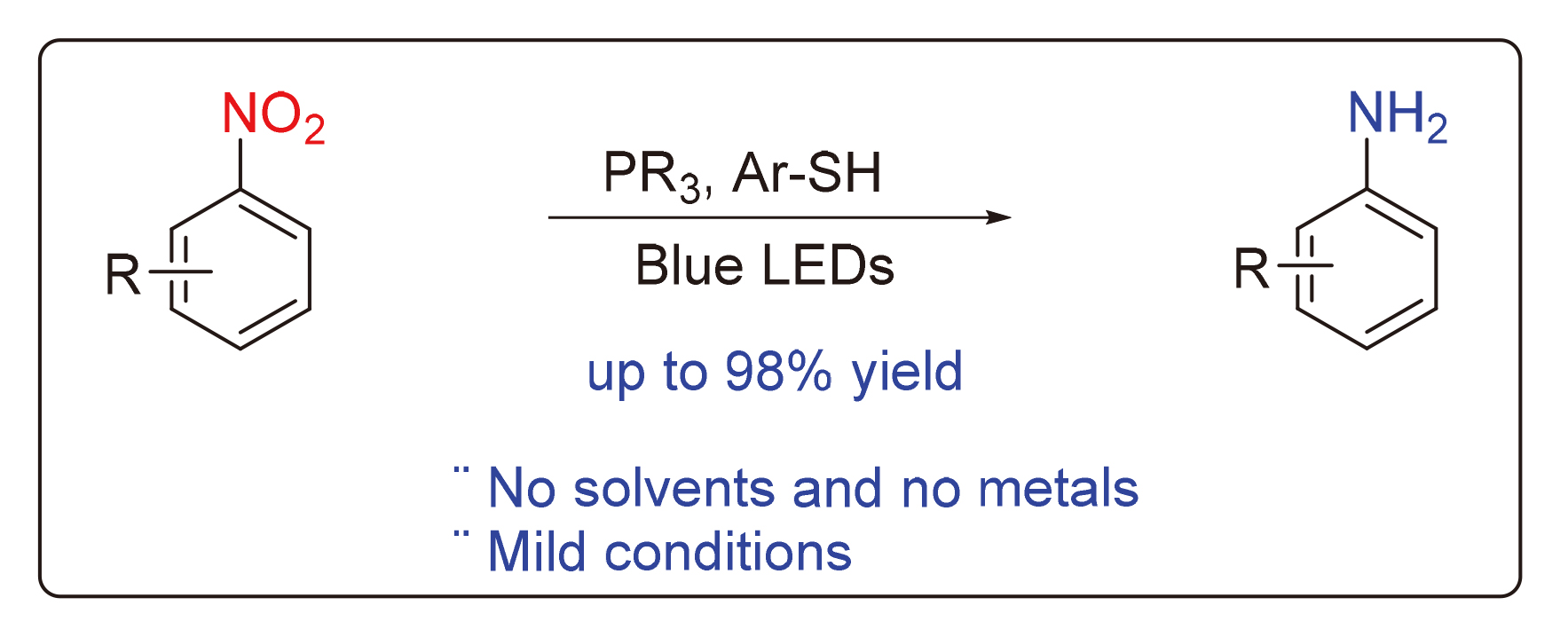
Key words: nitroaromatics; reduction; photochemistry; aromatic amines
| [1] | (a) Zhu, S.; Xu, S.; Jing, W.; Zhao, Z.; Jiang, J. J. Agric. Food Chem. 2016, 64, 9702. |
| [1] | (b) Liu, X. H.; Wen, Y. H.; Cheng, L.; Xu, T. M.; Wu, N. J. J. Agric. Food Chem. 2021, 69, 6968. |
| [2] | (a) Ye, L. J.; Toh, H. H.; Yang, Y.; Adams, J. P.; Snajdrova, R.; Li, Z. ACS Catal. 2015, 5, 1119. |
| [2] | (b) Werner, L.; Machara, A.; Hudlicky, T. Adv. Synth. Catal. 2010, 352, 195. |
| [2] | (c) Das Sarma, K.; Zhang, J.; Huang, Y.; Davidson, J. G. Eur. J. Org. Chem. 2006, 2006, 3730. |
| [3] | (a) Lin, C.-F.; Chien, C.-W.; Ojima, I. Org. Chem. Front. 2014, 1, 1062. |
| [3] | (b) Afanasyev, O. I.; Kuchuk, E.; Usanov, D. L.; Chusov, D. Chem. Rev. 2019, 119, 11857. |
| [4] | (a) Strotman, N. A.; Baxter, C. A.; Brands, K. M.; Cleator, E.; Krska, S. W.; Reamer, R. A.; Wallace, D. J.; Wright, T. J. J. Am. Chem. Soc. 2011, 133, 8362. |
| [4] | (b) Magnus, P.; Sane, N.; Fauber, B. P.; Lynch, V. J. Am. Chem. Soc. 2009, 131, 16045. |
| [4] | (c) Cox, C. D.; Breslin, M. J.; Whitman, D. B.; Schreier, J. D.; McGaughey, G. B.; Bogusky, M. J.; Roecker, A. J.; Mercer, S. P.; Bednar, R. A.; Lemaire, W.; Bruno, J. G.; Reiss, D. R.; Harrell, C. M.; Murphy, K. L.; Garson, S. L.; Doran, S. M.; Prueksaritanont, T.; Anderson, W. B.; Tang, C.; Roller, S.; Cabalu, T. D.; Cui, D.; Hartman, G. D.; Young, S. D.; Koblan, K. S.; Winrow, C. J.; Renger, J. J.; Coleman, P. J. J. Med. Chem. 2010, 53, 5320. |
| [4] | (d) Baxter, C. A.; Cleator, E.; Brands, K. M. J.; Edwards, J. S.; Reamer, R. A.; Sheen, F. J.; Stewart, G. W.; Strotman, N. A.; Wallace, D. J. Org. Process Res. Dev. 2011, 15, 367. |
| [5] | (a) Xu, D.-Q.; Hu, Z.-Y.; Li, W.-W.; Luo, S.-P.; Xu, Z.-Y. J. Mol. Catal. A: Chem. 2005, 235, 137. |
| [5] | (b) Wu, G.; Huang, M.; Richards, M.; Poirier, M.; Wen, X.; Draper, R. W. Synthesis 2003, 1657. |
| [5] | (c) Xu, S.; Tang, J.; Zhou, Q.; Du, J.; Li, H. ACS Sustainable Chem. Eng. 2019, 7, 16190. |
| [5] | (d) Xiao, Q.; Sarina, S.; Waclawik, E. R.; Jia, J.; Chang, J.; Riches, J. D.; Wu, H.; Zheng, Z.; Zhu, H. ACS Catal. 2016, 6, 1744. |
| [5] | (e) Tsutsumi, K.; Uchikawa, F.; Sakai, K.; Tabata, K. ACS Catal. 2016, 6, 4394. |
| [5] | (f) Park, Y.; Kim, Y.; Chang, S. Chem. Rev. 2017, 117, 9247. |
| [5] | (g) Pachisia, S.; Kishan, R.; Yadav, S.; Gupta, R. Inorg. Chem. 2021, 60, 2009. |
| [5] | (h) Liu, Y.; Miao, W.; Tang, W.; Xue, D.; Xiao, J.; Wang, C.; Li, C. Chem. Asian J. 2021, 16, 1725. |
| [5] | (i) Gutiérrez-Tarriño, S.; Rojas-Buzo, S.; Lopes, C. W.; Agostini, G.; Calvino, J. J.; Corma, A.; Oña-Burgos, P. Green Chem. 2021, 23, 4490. |
| [5] | (j) Cheung, C. W.; Hu, X. Nat. Commun. 2016, 7, 12494. |
| [5] | (k) Blaser, H.-U.; Steiner, H.; Studer, M. ChemCatChem 2009, 1, 210. |
| [6] | (a) Gao, Y.; Ma, D.; Wang, C.; Guan, J.; Bao, X. Chem. Commun. 2011, 47, 2432. |
| [6] | (b) Giomi, D.; Alfini, R.; Brandi, A. Tetrahedron 2011, 67, 167. |
| [6] | (c) Li, B.; Xu, Z. J. Am. Chem. Soc. 2009, 131, 16380. |
| [6] | (d) Orlandi, M.; Benaglia, M.; Tosi, F.; Annunziata, R.; Cozzi, F. J. Org. Chem. 2016, 81, 3037. |
| [6] | (e) Orlandi, M.; Tosi, F.; Bonsignore, M.; Benaglia, M. Org. Lett. 2015, 17, 3941. |
| [6] | (f) Sharma, S.; Kumar, M.; Kumar, V.; Kumar, N. J. Org. Chem. 2014, 79, 9433. |
| [7] | Pirola, M.; Faverio, C.; Orlandi, M.; Benaglia, M. Chem.-Eur. J. 2021, 27, 10247. |
| [8] | Porwal, D.; Oestreich, M. Eur. J. Org. Chem. 2016, 2016, 3307. |
| [9] | Freeman, A. W.; Urvoy, M.; Criswell, M. E. J. Org. Chem. 2005, 70, 5014. |
| [10] | Duan, Z.; Ranjit, S.; Liu, X. Org. Lett. 2010, 12, 2430. |
| [11] | Shi, J.; Wei, W. Chin. J. Org. Chem. 2020, 40, 2170. (in Chinese) |
| [11] | ( 时建伟, 魏伟, 有机化学, 2020, 40, 2170.) |
| [12] | (a) Peng, S.; Lin, Y.; He, W.-M. Chin. J. Org. Chem. 2020, 40, 541. (in Chinese) |
| [12] | ( 彭莎, 林英武, 何卫民, 有机化学, 2020, 40, 541.) |
| [12] | (b) Chen, D.; Sun, Y.; Dong, D.; Han, Q.; Wang, Z. Chin. J. Org. Chem. 2020, 40, 4267. (in Chinese) |
| [12] | ( 陈德茂, 孙媛媛, 董道青, 韩晴晴, 王祖利, 有机化学, 2020, 40, 4267.) |
| [12] | (c) Ge, L.; Chiou, M.-F.; Li, Y.; Bao, H. Green Synth. Catal. 2020, 1, 86. |
| [12] | (d) Cao, D.; Pan, P.; Li, C.-J.; Zeng, H. Green Synth. Catal. 2021, 2, 303. |
| [12] | (e) Wang, P.; Zhao, Q.; Xiao, W.; Chen, J. Green Synth. Catal. 2020, 1, 42. |
| [12] | (f) Tong, S.; Li, K.; Ouyang, X.; Song, R.; Li, J. Green Synth. Catal. 2021, 2, 145. |
| [13] | (a) Wang, Z.; Liu, Q.; Liu, R.; Ji, Z.; Li, Y.; Zhao, X.; Wei, W. Chin. Chem. Lett. 2021 DOI: 10.1016/j.cclet.2021.08.036. |
| [13] | (b) Meng, N.; Lv, Y.; Liu, Q.; Liu, R.; Zhao, X.; Wei, W. Chin. Chem. Lett. 2021, 32, 258. |
| [13] | (c) Wang, L.; Bao, P.; Liu, W.; Liu, S.; Hu, C.; Yue, H.; Yang, D.; Wei, W. Chin. J. Org. Chem. 2018, 38, 3189. (in Chinese) |
| [13] | ( 王雷雷, 鲍鹏丽, 刘维伟, 刘思彤, 胡昌松, 岳会兰, 杨道山, 魏伟, 有机化学, 2018, 38, 3189.) |
| [13] | (d) Bao, P.; Liu, F.; Lv, Y.; Yue, H.; Li, J.-S.; Wei, W. Org. Chem. Front. 2020, 7, 492. |
| [14] | (a) Jin, W.; Liu, C. Chin. J. Org. Chem. 2021, 41, 2148. (in Chinese) |
| [14] | ( 金伟伟, 刘晨江, 有机化学, 2021, 41, 2148.) |
| [14] | (b) Gui, Q.-W.; Teng, F.; Li, Z.-C.; Xiong, Z.-Y.; Jin, X.-F.; Lin, Y.-W.; Cao, Z.; He, W.-M. Chin. Chem. Lett. 2021, 32, 1907. |
| [14] | (c) Sun, K.; Xiao, F.; Yu, B.; He, W.-M. Chin. J. Catal. 2021, 42, 1921. |
| [15] | Qu, Z.; Chen, X.; Zhong, S.; Deng, G. J.; Huang, H. Org. Lett. 2021, 23, 5349. |
| [16] | Smolinsky, G.; Feuer, B. I. J. Org. Chem. 1966, 31, 3882. |
| [17] | Zhao, L.; Hu, C.; Cong, X.; Deng, G.; Liu, L. L.; Luo, M.; Zeng, X. J. Am. Chem. Soc. 2021, 143, 1618. |
| [18] | Li, J.; Shi, X.-Y.; Bi, Y.-Y.; Wei, J.-F.; Chen, Z.-G. ACS Catal. 2011, 1, 657. |
| [19] | Wienhofer, G.; Sorribes, I.; Boddien, A.; Westerhaus, F.; Junge, K.; Junge, H.; Llusar, R.; Beller, M. J. Am. Chem. Soc. 2011, 133, 12875. |
| [20] | Yao, W.; Wang, J.; Lou, Y.; Wu, H.; Qi, X.; Yang, J.; Zhong, A. Org. Chem. Front. 2021, 8, 4554. |
| [21] | Formenti, D.; Ferretti, F.; Topf, C.; Surkus, A.-E.; Pohl, M.-M.; Radnik, J.; Schneider, M.; Junge, K.; Beller, M.; Ragaini, F. J. Catal. 2017, 351, 79. |
| [22] | Lu, H.; Geng, Z.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Org. Lett. 2016, 18, 2774. |
/
| 〈 |
|
〉 |