

过氧化氢原位生成驱动过氧合酶催化反应研究进展
收稿日期: 2021-08-28
修回日期: 2021-09-30
网络出版日期: 2021-11-17
基金资助
国家自然科学基金(32171253)
Research Progress of Peroxygenase-Catalyzed Reactions Driven by in-situ Generation of H2O2
Received date: 2021-08-28
Revised date: 2021-09-30
Online published: 2021-11-17
Supported by
National Natural Science Foundation of China(32171253)
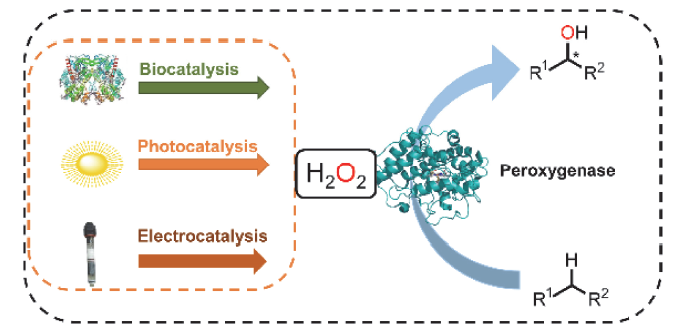
C—H键的选择性催化氧官能团化反应是有机化学中非常具有挑战的一类反应. 与贵金属络合物催化剂相比, 生物酶在选择性和活性等方面表现出一定优势. 其中, 过氧合酶(peroxygenase)作为近年来研究热点, 能够直接利用过氧化氢(H2O2)作为共底物催化C—H键的氧官能团化反应. 过氧合酶结合了P450单加氧酶的催化多功能性且无须依赖辅酶及其再生体系, 因此在有机合成中引起越来越多的重视. 但是, 过氧合酶在实际应用中存在一个瓶颈: 即过氧合酶对其催化所必须的H2O2浓度敏感, 当反应体系中H2O2浓度过高时, 会引起过氧合酶中的亚铁血红素氧化分解, 从而导致酶失活. 为突破该瓶颈, 调控反应体系中H2O2的浓度对于高效应用过氧合酶来说格外重要. 概括了近年来多种原位生成H2O2驱动过氧合酶催化的方法. 从酶催化、光催化、电催化原位生成H2O2角度出发, 全面分析了近年来开发的高原子经济性H2O2原位生成技术驱动过氧合酶催化体系的优缺点, 为促进该酶在有机合成中的应用提供参考.
李可欣 , 杨庆远 , 张鹏鹏 , 张武元 . 过氧化氢原位生成驱动过氧合酶催化反应研究进展[J]. 有机化学, 2022 , 42(3) : 732 -741 . DOI: 10.6023/cjoc202108052
Selective catalytic oxygen functionalization reactions of C—H bonds are a very challenging class of reactions in organic chemistry. Compared with tranditional metal complex catalysts, enzymes exhibit certain advantages in terms of selectivity and activity. Amongest them, peroxygenases are able to catalyze the oxidative functionalization of C—H bonds by direct use of H2O2 as a co-substrate. Peroxygenases combine the catalytic versatility of P450 monooxygenases without relying on coenzymes and their regenerative systems for cofactors. Therefore, peroxygenases have attracted considerable attention in organic synthesis. However, a bottleneck in the practical application of peroxygenase is that, like all heme-dependent enzymes, peroxygenase is sensitive to H2O2, and higher concentration of H2O2 in the reaction system will cause oxidative decomposition of ferrous heme, resulting in the enzyme inactivation. To overcome this, regulating the concentration of H2O2 in the reaction is particularly important for the efficient use of peroxygenase. This paper outlines a variety of methods used for in situ generation of H2O2 to drive peroxygenases reported in recent years. The pros and cons of biocatalysis, photocatalysis and electrocatalysis used for high atom-efficient H2O2 generation are comprehensively analyzed. This review paper is expected to provide a reference for promoting the application of peroxygenases in organic synthesis.

| [1] | Dong, J.-J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C. E.; Pasic, M.; Schmidt, S.; Wang, Y.-H.; Younes, S.; Zhang, W.-Y. Angew. Chem., Int. Ed. 2018, 57, 9238. |
| [2] | Zeng, Z.-G.; Sang, X.-K.; Yuan, B.; Wu, M.-H.; Zhang, W.-Y. Chin. J. Org. Chem. 2021, 41, 959. (in Chinese) |
| [2] | (曾志刚, 桑贤轲, 袁波, 吴鸣虎, 张武元, 有机化学, 2021, 41, 959.) |
| [3] | Bormann, S.; Baraibar, A. G.; Ni, Y.; Holtmann, D.; Hollmann, F. Catal. Sci. Technol. 2015, 5, 2038. |
| [4] | Ullrich, R.; Nüske, J.; Scheibner, K.; Spantzel, J.; Hofrichter, M. Appl. Environ. Microbiol. 2004, 70, 4575. |
| [5] | Hofrichter, M.; Ullrich, R. Appl. Microbiol. Biotechnol. 2006, 71, 276. |
| [6] | Zhang, W.-Y.; Hollmann, F. Synthesis of Vinyl Polymers via Enzymatic Oxidative Polymerisation, Springer Nature Singapore Pte Ltd, Singapore, 2019, p. 343. |
| [7] | Peter, S.; Kinne, M.; Wang, X.-S.; Ullrich, R.; Kayser, G.; Groves, J. T.; Hofrichter, M. FEBS J. 2011, 278, 3667. |
| [8] | Lucas, F.; Babot, E. D.; Cañellas, M.; del Río, J. C.; Kalum, L.; Ullrich, R.; Hofrichter, M.; Guallar, V.; Martínez, A. T.; Gutiérrez, A. Catal. Sci. Technol. 2016, 6, 288. |
| [9] | Kinne, M.; Ullrich, R.; Hammel, K. E.; Scheibner, K.; Hofrichter, M. Tetrahedron Lett. 2008, 49, 5950. |
| [10] | de Santons, P. G.; Cañellas, M.; Tieves, F.; Younes, S. H. H.; Molina-Espeja, P.; Hofrichter, M.; Hollmann, F.; Guallar, V.; Alcalde, M. ACS Catal. 2018, 8, 4789. |
| [11] | Molina-Espeja, P.; Cañellas, M.; Plou, F. J.; Hofrichter, M.; Lucas, F.; Guallar, V.; Alcalde, M. ChemBioChem 2016, 17, 341. |
| [12] | Thiel, D.; Doknić, D.; Deska, J. Nat. Commun. 2014, 5, 5278. |
| [13] | Valderrama, B.; Ayala, M.; Vazquez-Duhalt, R. Chem. Biol. 2002, 9, 555. |
| [14] | van de Velde, F.; Lourenco, N. D.; Bakker, M.; van Rantwijk, F.; Sheldon, R. A. Biotechnol. Bioeng. 2000, 69, 286. |
| [15] | Bakker, M.; van de Velde, F.; van Rantwijk, F.; Sheldon, R. A. Biotechnol. Bioeng. 2000, 70, 342. |
| [16] | Okrasa, K.; Guibé-Jampel, E.; Therisod, M. J. Chem. Soc., Perkin Trans. 1 2000, 7, 1077. |
| [17] | Ribitsch, D.; Karl, W.; Wehrschütz-Sigl, E.; Tutz, S.; Remler, P.; Weber, H. J.; Gruber, K.; Stehr, R.; Bessler, C.; Hoven, N.; Sauter, K.; Schwab, H. Appl. Microbiol. Biotechnol. 2009, 81, 875. |
| [18] | Okrasa, K.; Falcimaigne, A.; Guibé-Jampel, E.; Therisod, M. Tetrahedron: Asymmetry 2002, 13, 519. |
| [19] | Pezzotti, F.; Okrasa, K.; Therisod, M. Tetrahedron: Asymmetry 2005, 16, 2681. |
| [20] | Pezzotti, F.; Therisod, M. Tetrahedron: Asymmetry 2007, 18, 701. |
| [21] | Rocha-Martin, J.; Velasco-Lozano, S.; Guisán, J. M.; López- Gallego, F. Green Chem. 2014, 16, 303. |
| [22] | Jung, D.; Streb, C.; Hartmann, M. Microporous Mesoporous Mater. 2008, 113, 523. |
| [23] | Ni, Y.; Fernández-Fueyo, E.; Baraibar, A. G.; Ullrich, R.; Hofrichter, M.; Yanase, H.; Alcalde, M.; van Berkel, W. J. H.; Hollmann, F. Angew. Chem., Int. Ed. 2015, 55, 798. |
| [24] | Chang, A.; Scheer, M.; Grote, A.; Schomburg, I.; Schomburg, D. Nucleic Acids Res. 2009, 37, D588. |
| [25] | Pesic, M; Willot, S. J. P.; Fernández-Fueyo, E.; Tieves, F.; Alcalde, M.; Hollamnn, F. Z. Naturforsch., C: J. Biosci. 2018, 74, 100. |
| [26] | Tieves, F.; Willot, S. J. P.; van Schie, M. M. C. H.; Rauch, M. C. R.; Younes, S. H. H.; Zhang, W.-Y.; Dong, J.-J.; de Santos, P. G.; Robbins, J. M.; Bommarius, B.; Alcalde, M.; Bommarius, A. S.; Hollmann, F. Angew. Chem., Int. Ed. 2019, 58, 7873. |
| [27] | Willot, S. J. P.; Hoang, M. D.; Paul, C. E.; Alcalde, M.; Arends, I. W. C. E.; Bommarius, A. S.; Bommarius, B.; Hollmann, F. ChemCatChem 2020, 12, 2713. |
| [28] | Li, Y.-Y.; Yuan, B.; Sun, Z.-T.; Zhang, W.-Y. Green Synth. Catal. 2021, 2, 267. |
| [29] | Perez, D. I.; Grau, M. M.; Arends, I. W. C. E.; Hollmann, F. Chem. Commun. 2009, 41, 6848. |
| [30] | Churakova, E.; Kluge, M.; Ullrich, R.; Arends, I.; Hofrichter, M.; Hollmann, F. Angew. Chem., Int. Ed. 2011, 50, 10716. |
| [31] | Churakova, E.; Arends, I. W. C. E.; Hollmann, F. ChemCatChem 2013, 5, 565. |
| [32] | Zhang, W.-Y.; Burek, B. O.; Fernández-Fueyo, E.; Alcalde, M.; Bloh, J. Z.; Hollmann, F. Angew. Chem. 2017, 56, 15451. |
| [33] | Zhang, W.-Y.; Fernández-Fueyo, E.; Ni, Y.; van Schie, M.; Gacs, J.; Renirie, R.; Wever, R.; Mutti, F. G.; Rother, D. R.; Alcalde, M.; Hollmann, F. Nat. Catal. 2018, 1, 55. |
| [34] | Teranishi, M.; Hoshino, R.; Naya, S.; Tada, H. Angew. Chem. 2016, 128, 12965. |
| [35] | van Schie, M. M. C. H.; Zhang, W.-Y.; Tieves, F.; Choi, D. S.; Park, C. B.; Burek, B. O.; Bloh, J. Z.; Arends, I. W. C. E.; Paul, C. E.; Alcalde, M.; Hollmann, F. ACS Catal. 2019, 9, 7409. |
| [36] | Willot, S. J. P.; Fernández-Fueyo, E.; Tieves, F.; Pesic, M.; Alcalde, M.; Arends, I. W. C. E.; Park, C. B.; Hollmann, F. ACS Catal. 2019, 9, 890. |
| [37] | Yuan, B.; Mahor, D.; Fei, Q.; Wever, R.; Alcalde, M.; Zhang, W.-Y.; Hollmann, F. ACS Catal. 2020, 10, 8277. |
| [38] | Zhang, W.-Y.; Liu, H.-H.; van Schie, M. M. C. H.; Hagedoorn, P. L.; Alcalde, M.; Denkova, A. G.; Djanashvili, K.; Hollmann, F. ACS Catal. 2020, 10, 14195. |
| [39] | Lvtz, S.; Steckhan, E.; Liese, A. Electrochem. Commun. 2004, 6, 583. |
| [40] | Kohlmann, C.; Lvtz, S. Eng. Life Sci. 2006, 6, 170. |
| [41] | Lvtz, S.; Vuorilehto, K.; Liese, A. Biotechnol. Bioeng. 2007, 98, 525. |
| [42] | Horst, A. E. M.; Mangold, K. M.; Holtmann, D. Biotechnol. Bioeng. 2016, 113, 260. |
| [43] | Krieg, T.; Hvttmann, S.; Mangold, K. M.; Schrader, J.; Holtmann, D. Green Chem. 2011, 13, 2686. |
| [44] | Holtmann, D.; Krieg, T.; Getrey, L.; Schrader, J. Catal. Commun. 2014, 51, 82. |
| [45] | Horst, A. E. W.; Bormann, S.; Meyer, J.; Steinhagen, M.; Ludwig, R.; Drews, A.; Ansorge-Schumacher, M.; Holtmann, D. J. Mol. Catal. B: Enzym. 2016, 133, S137. |
| [46] | Choi, D. S.; Ni, Y.; Fernández-Fueyo, E.; Lee, M.; Hollmann, F.; Park, C. B. ACS Catal. 2017, 7, 1563. |
| [47] | Kim, H. W.; Ross, M. B.; Kornienko, N.; Zhang, L.; Guo, J.-H.; Yang, P.-D.; McCloskey, B. D. Nat. Catal. 2018, 1, 282. |
| [48] | Bormann, S.; van Schie, M. M. C. H.; De Almeida, T. P.; Zhang, W.-Y.; Stöckl, M.; Ulber, R.; Hollmann, F.; Holtmann, D. ChemSusChem 2019, 12, 4759. |
| [49] | Karmee, S. K.; Roosen, C.; Kohlmann, C.; Lvtz, S.; Greiner, L.; Leitner, W. Green Chem. 2009, 11, 1052. |
| [50] | Edwards, J. K.; Freakley, S. J.; Carley, A. F.; Kiely, C. J.; Hutchings, G. J. Acc. Chem. Res. 2014, 47, 845. |
| [51] | Freakley, S. J.; Kochius, S.; van Marwijk, J.; Fenner, C.; Lewis, R. J.; Baldenius, K.; Marais, S. S.; Opperman, D. J.; Harrison, S. T. L.; Alcalde, M.; Smit, M. S.; Hutchings, G. J. Nat. Commun. 2019, 10, 1. |
| [52] | Yoon, J.; Kim, J.; Tieves, F.; Zhang, W.-Y.; Alcalde, M.; Hollmann, F.; Park, C. B. ACS Catal. 2020, 10, 5236. |
/
| 〈 |
|
〉 |