

砜亚胺N-芳基化的研究进展及其应用
收稿日期: 2021-10-09
修回日期: 2021-11-02
网络出版日期: 2021-11-25
基金资助
深圳市科技创新委员会(JCYJ20180302180256215)
Recent Advances in N-Arylation of NH-Sulfoximines and Their Applications
Received date: 2021-10-09
Revised date: 2021-11-02
Online published: 2021-11-25
Supported by
Science and Technology Innovation Commission of Shenzhen Municipality(JCYJ20180302180256215)
李雪 , 王聪 , 贾铁争 . 砜亚胺N-芳基化的研究进展及其应用[J]. 有机化学, 2022 , 42(3) : 714 -731 . DOI: 10.6023/cjoc202110011
Sulfoximines represent an important structural motif in organic chemistry, and have been utilized as chiral auxiliaries, chiral ligands and organocatalysts. They also serve as key intermediates for the construction of heterocyclic compounds. Attributing to their unique bioactivities, sulfoximines have been developed as previliged pharmacophores and widely used in pharmaceutical chemistry and agriculture. Considering the wide applications, the synthetic methods to afford sulfoximines have attracted increasing attention. Among them, the direct arylation of NH-sulfoximines to prepare NAr- sulfoxmines exhibites some unique advantages, including atom-economics, mild conditions and step-economics, and thus has made tremedous progress in recent years. Various methods of NH-sulfoximines to afford NAr-sulfoximines via a C—N bond formation strategy, as well as their applications in synthesis of bioactive molecules and ligands for transition-metal catalysts, are reviewed.
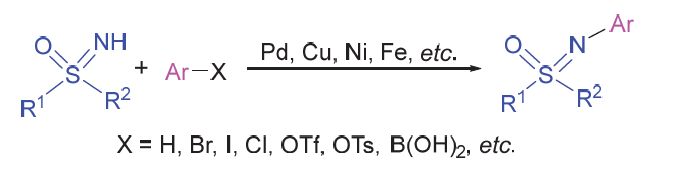
Key words: NH-sulfoximine; N-arylation; NAr-sulfoximine; C—N bond formation
| [1] | Lücking, U. Angew. Chem., Int. Ed. 2013, 52, 9399. |
| [2] | Lücking, U.; Jautelat, R.; Krüger, M.; Brumby, T.; Lienau, P.; Schäfer, M.; Briem, H.; Schulze, J.; Hillisch, A.; Reichel, A.; Wengner, A. M.; Siemeister, G. ChemMedChem 2013, 8, 1021. |
| [3] | Zhu, Y.; Loso, M. R.; Watson, G. B.; Sparks, T. C.; Rogers, R. B.; Huang, J. X.; Gerwick, B. C.; Babcock, J. M.; Kelley, D.; Hegde, V. B.; Nugent, B. M.; Renga, J. M.; Denholm, I.; Gorman, K.; DeBoer, G. J.; Hasler, J.; Meade, T.; Thomas, J. D. J. Agric. Food Chem. 2011, 59, 2950. |
| [4] | (a) Bizet, V.; Hendriks, C. M.; Bolm, C. Chem. Soc. Rev. 2015, 44, 3378. |
| [4] | (b) Schafer, S.; Wirth, T. Angew. Chem., Int. Ed. 2010, 49, 2786. |
| [4] | (c) Tota, A.; Zenzola, M.; Chawner, S. J.; John-Campbell, S. S.; Carlucci, C.; Romanazzi, G.; Degennaro, L.; Bull, J. A.; Luisi, R. Chem. Commun. 2016, 53, 348. |
| [5] | Bolm, C.; Hildebrand, J. P. Tetrahedron Lett. 1998, 39, 5731. |
| [6] | Bolm, C.; Hildebrand, J. P. J. Org. Chem. 2000, 65, 169. |
| [7] | Harmata, M.; Hong, X. Synlett 2007, 6, 969. |
| [8] | Yongpruksa, N.; Calkins, N. L.; Harmata, M. Chem. Commun. 2011, 47, 7665. |
| [9] | Yang, Q.; Choy, P. Y.; Zhao, Q.; Leung, M. P.; Chan, H. S.; So, C. M.; Wong, W. T.; Kwong, F. Y. J. Org. Chem. 2018, 83, 11369. |
| [10] | Cho, G. Y.; Remy, P.; Jansson, J.; Moessner, C.; Bolm, C. Org. Lett. 2004, 6, 3293. |
| [11] | Sedelmeier, J.; Bolm, C. J. Org. Chem. 2005, 70, 6904. |
| [12] | Vaddula, B.; Leazer, J.; Varma, R. S. Adv. Synth. Catal. 2012, 354, 986. |
| [13] | Miyasaka, M.; Hirano, K.; Satoh, T.; Kowalczyk, R.; Bolm, C.; Miura, M. Org. Lett. 2011, 13, 359. |
| [14] | Wang, L.; Priebbenow, D. L.; Dong, W.; Bolm, C. Org. Lett. 2014, 16, 2661. |
| [15] | Grandhi, G. S.; Dana, S.; Mandal, A.; Baidya, M. Org. Lett. 2020, 22, 2606. |
| [16] | Moessner, C.; Bolm, C. Org. Lett. 2005, 7, 2667. |
| [17] | Gupta, S.; Baranwal, S.; Muniyappan, N.; Sabiah, S.; Kandasamy, J. Synthesis 2019, 51, 2171. |
| [18] | Wang, C.; Zhang, H.; Wells, L. A.; Liu, T.; Meng, T.; Liu, Q.; Walsh, P. J.; Kozlowski, M. C.; Jia, T. Nat. Commun. 2021, 12, 932. |
| [19] | Kim, J.; Ok, J.; Kim, S.; Choi, W.; Lee, P. H. Org. Lett. 2014, 16, 4602. |
| [20] | Zhu, H.; Teng, F.; Pan, C.; Cheng, J.; Yu, J.-T. Tetrahedron Lett. 2016, 57, 2372. |
| [21] | Hande, S.; Mfuh, A.; Throner, S.; Wu, Y.; Ye, Q.; Zheng, X. Tetrahedron Lett. 2019, 60, 151100. |
| [22] | Wimmer, A.; König, B. Org. Lett. 2019, 21, 2740. |
| [23] | Liu, D.; Liu, Z. R.; Ma, C.; Jiao, K. J.; Sun, B.; Wei, L.; Lefranc, J.; Herbert, S.; Mei, T. S. Angew. Chem., Int. Ed. 2021, 60, 9444. |
| [24] | Correa, A.; Bolm, C. Adv. Synth. Catal. 2008, 350, 391. |
| [25] | Wimmer, A.; König, B. Adv. Synth. Catal. 2018, 360, 3277. |
| [26] | Aithagani, S. K.; Dara, S.; Munagala, G.; Aruri, H.; Yadav, M.; Sharma, S.; Vishwakarma, R. A.; Singh, P. P. Org. Lett. 2015, 17, 5547. |
| [27] | Meier, R.; Hog, D.; Lämmermann, H.; Sudau, A.; Rackl, D.; Weinmann, H.; Collins, K.; Wortmann, L.; Candish, L. Synlett 2018, 29, 2679. |
| [28] | Harmata, M.; Pavri, N. Angew. Chem., Int. Ed. 1999, 38, 2577. |
| [29] | Harmata, M.; Ghosh, S. K. Org. Lett. 2001, 3, 3321. |
| [30] | Bolm, C.; Simic, O. J. Am. Chem. Soc. 2001, 123, 3830. |
| [31] | Bolm, C.; Martin, M.; Simic, O.; Verrucci, M. Org. Lett. 2003, 5, 427. |
| [32] | Langner, M.; Bolm, C. Angew. Chem., Int. Ed. 2004, 43, 5984. |
| [33] | Langner, M.; Remy, P.; Bolm, C. Chem.-Eur. J. 2005, 11, 6254. |
| [34] | Frings, M.; Atodiresei, I.; Wang, Y.; Runsink, J.; Raabe, G.; Bolm, C. Chem.-Eur. J. 2010, 16, 4577. |
| [35] | Moessner, C.; Bolm, C. Angew. Chem., Int. Ed. 2005, 44, 7564. |
| [36] | Biosca, M.; P?mies, O.; Diéguez, M. J. Org. Chem. 2019, 84, 8259. |
| [37] | Harmata, M.; Hong, X. J. Am. Chem. Soc. 2003, 125, 5754. |
| [38] | Harmata, M.; Hong, X. Org. Lett. 2007, 9, 2701. |
| [39] | Battula, S. R. K.; Subbareddy, G. V.; Chakravarthy, I. E.; Saravanan, V. RSC Adv. 2016, 6, 55710. |
/
| 〈 |
|
〉 |