

单质硫: 合成含硫杂环的优质硫源
收稿日期: 2021-09-24
网络出版日期: 2021-12-08
基金资助
河北省高等学校科学技术研究(ZD2020153); 河北省省属高等学校基本科研业务费研究(XKF202102); 大中学生科技创新能力培育专项(2021H100403)
Elemental Sulfur: An Excellent Sulfur-Source for Synthesis of Sulfur-Containing Heterocyclics
Received date: 2021-09-24
Online published: 2021-12-08
Supported by
Science and Technology Project of Hebei Education Department(ZD2020153); Fundamental Research Funds for the Universities in Hebei Province(XKF202102); Special Project of Cultivating College Students Scientific and Technological Innovation Ability(2021H100403)
肖立伟 , 刘光仙 , 任萍 , 吴彤桐 , 卢玉伟 , 孔洁 . 单质硫: 合成含硫杂环的优质硫源[J]. 有机化学, 2022 , 42(4) : 1002 -1012 . DOI: 10.6023/cjoc202109038
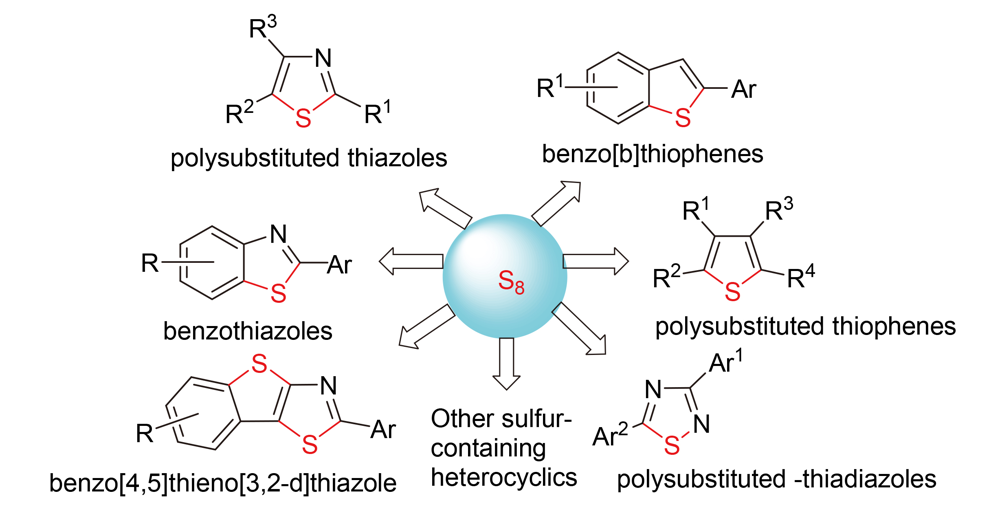
Sulfur-containing heterocyclic compounds are widely applied in many areas such as drugs, molecular devices and materials, etc., so their synthesis methods have attracted much attention. The key step for the synthesis of sulfur-containing heterocycles is the construction of C—S bond, which is generally realized by C—H bond functionalization or C—X bond coupling reaction with sulfur-containing organic or inorganic substances. Elemental sulfur is cheap, available and stable, and it is an excellent sulfur atom donor for the synthesis of sulfur-containing heterocycles. Therefore, the synthesis of sulfur-containing heterocycles from elemental sulfur has become a research hotspot. Herein, the recent research progress of synthesis of sulfur-containing heterocyclic employing sulfur as sulfur-source is reviewed.
| [1] | (a) Cui, S. F.; Wan, Y.; Lv, J. S.; Damu, G. L. V.; Zhou, C. H. Sci. China Chem. 2012, 42, 1105. (in Chinese) |
| [1] | ( 崔胜峰, 王艳, 吕敬松, Damu, Guri, L. V.; 周成合; 中国科学: 化学, 2012, 42, 1105.) |
| [1] | (b) He, W.; Liu, D.; Gan, X.; Zhang, J.; Liu, Z.; Yi, C.; Song, B. Chin. J. Org. Chem. 2019, 39, 2287. (in Chinese) |
| [1] | ( 何文静, 刘登曰, 甘秀海, 张建, 刘峥军, 易崇粉, 宋宝安, 有机化学. 2019, 39, 2287.) |
| [2] | (a) Xiao, L. W.; Xiao, S. Q.; Li, Z.; Jing, X. M.; Dai, F. C. Chemistry 2019, 82, 120. (in Chinese) |
| [2] | ( 肖立伟, 肖树强, 李政, 景学敏, 戴富才, 化学通报, 2019, 82, 120.) |
| [2] | (b) Xiao, L. W.; Liu, G. X.; Jing, X. M.;Ren L. L.; Xiao, S. Q. Chem. Res. Appl. 2020, 32, 297. (in Chinese) |
| [2] | ( 肖立伟, 刘光仙, 景学敏, 任丽磊, 肖树强, 化学研究与应用, 2020, 32, 297.) |
| [3] | (a) Beletskaya, I. P.; Ananikov, V. P. Chem. Rev. 2011, 111, 1596. |
| [3] | (b) Yang, W.; Zhang, M.; Chen, W.; Yang, X.; Feng, J. Chin. J. Org. Chem. 2020, 40, 4060. (in Chinese) |
| [3] | ( 杨文超, 张明明, 陈旺, 杨小虎, 冯建国, 有机化学, 2020, 40, 4060.) |
| [4] | (a) Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T. S. A.; Liu, X. Chem. Soc. Rev. 2015, 44, 291. |
| [4] | (b) Sundaravelu, N.; Sangeetha, S.; Sekar, G. Org. Biomol. Chem. 2021, 19, 1459. |
| [5] | (a) Nguyena, T. B. Adv. Synth. Catal. 2017, 359, 1066. |
| [5] | (b) Li, J.; Yang, S.; Wu, W.; Jiang, H. Org. Chem. Front. 2020, 7, 1395. |
| [5] | (c) Nguyen, T. B. Adv. Synth. Catal. 2020, 362, 3448. |
| [5] | (d) Fan, R.; Tan, C.; Liu, Y.; Wei, Y.; Zhao, X.; Liu, X.; Tan, J.; Yoshida, H. Chin. Chem. Lett. 2021, 32, 299. |
| [5] | (e) Cheng, H.; Guo, P.; Chen, B.; Yao, J.; Ma, J.; Hu, W.; Ji, H. Chin. J. Org. Chem. 2021, 41, 94. (in Chinese) |
| [5] | ( 程辉成, 郭鹏虎, 陈冰, 姚嘉伟, 马姣丽, 胡炜杰, 纪红兵, 有机化学, 2021, 41, 94.) |
| [6] | (a) Colebourne, N.; Foster, R. G.; Robson, E. J. Chem. Soc. C 1967, 685. |
| [6] | (b) Beck, G.; Heitzer, H.; Holtschmidt, H. Synthesis 1985, 586. |
| [6] | (c) Gewald, V. K.; Böttcher, H.; Mayer, R. J. Prakt. Chem. 1964, 23, 298. |
| [6] | (d) Gewald, V. K.; Spies, H.; Mayer, R. J. Prakt. Chem. 1970, 312, 776. |
| [7] | Deng, H.; Li, Z.; Ke, F.; Zhou, X. Chem.-Eur. J. 2012, 18, 4840. |
| [8] | Yang, Z.; Hu, R.; Li, X.; Wang, X.; Gu, R.; Han, S. Tetrahedron Lett. 2017, 58, 2366. |
| [9] | Wang, R.; Ding, Y. l.; Liu, H.; Peng, S.; Ren, J.; Li, L. Tetrahedron Lett. 2014, 55, 945. |
| [10] | Xu, H.; Luo, C.; Li, Z.; Xiang, H.; Zhou, X. J. Heterocycl. Chem. 2015, 53, 120. |
| [11] | Huang, Y.; Yan, D.; Wang, X.; Zhou, P.; Wu, W.; Jiang, H. Chem. Commun. 2018, 54, 1742. |
| [12] | Huang, Y.; Zhou, P.; Wu, W.; Jiang, H. J. Org. Chem. 2018, 83, 2460. |
| [13] | Pan, L.; Yu, L.; Wu, Z.; Li, Z.; Xiang, H.; Zhou, X. RSC Adv. 2015, 46, 27775. |
| [14] | Zhang, L. F.; Ni, Z. H.; Li, D. Y.; Qin, Z. H.; Wei, X. Y. Chin. Chem. Lett. 2012, 23, 281. |
| [15] | Li, G.; Xie, H.; Chen, J.; Guo, Y.; Deng, G. J. Green Chem. 2017, 19, 4043. |
| [16] | Che, X.; Jiang, J.; Xiao, F.; Huang, H.; Deng, G. J. Org. Lett. 2017, 19, 4576. |
| [17] | Deng, G.; Jiang, J.; Li, G.; Zhang, F.; Xie, H. Adv. Synth. Catal. 2018, 360, 1622. |
| [18] | Zhang, J.; Zhao, X.; Liu, P.; Sun, P. J. Org. Chem. 2019, 84, 12596. |
| [19] | Zhu, X.; Zhou, F.; Yang, Y.; Deng, G.; Liang, Y. ACS Omega 2020, 5, 22, 13136. |
| [20] | Liu, Y.; Yuan, X.; Guo, X.; Zhang, X.; Chen, B. Tetrahedron 2018, 74, 6057. |
| [21] | Huynh, T. V.; Doan, K. V.; Luong, N. T. K.; Nguyen, D. T. P.; Doan, S. H.; Nguyen, T. T.; Phan, N. T. S. RSC Adv. 2020, 10, 18423. |
| [22] | Singh, M.; Vaishali, Paul, A. K.; Singh, V. Org. Biomol. Chem. 2020, 18, 4459. |
| [23] | Zhu, X.; Yang, Y.; Xiao, G.; Song, J.; Liang, Y.; Deng, G. Chem. Commun. 2017, 53, 11917. |
| [24] | Fu, R. G.; Wang, Y.; Xia, F.; Zhang, H. L.; Sun, Y.; Yang, D. W.; Wang, Y. W.; Yin, P. J. Org. Chem. 2019, 84, 12237. |
| [25] | Nguyen, T. B.; Ermolenko, L.; Al-Mourabit, A. Org. Lett. 2013, 15, 4218. |
| [26] | Teramoto, M.; Imoto, M.; Takeda, M.; Mizuno, T.; Nomoto, A.; Ogawa, A. J. Org. Chem. 2020, 85, 15213. |
| [27] | Nguyen, L. A.; Ngo, Q. A.; Retailleau, P.; Nguyen, T. B. Green Chem. 2017, 19, 4289. |
| [28] | Nguyen, T. B.; Ermolenko, L.; Retailleau, P.; Al-Mourabit, A. Angew. Chem., Int. Ed. 2014, 53, 13808. |
| [29] | Tong, Y.; Pan, Q.; Jiang, Z.; Miao, D.; Shi, X.; Han, S. Tetrahedron Lett. 2014, 55, 5499. |
| [30] | Nguyen, T. B.; Pasturaud, K.; Ermolenko, L.; Al-Mourabit, A. Org. Lett. 2015, 17, 2562. |
| [31] | Guntreddi, T.; Vanjari, R.; Singh, K. N. Org. Lett. 2015, 17, 976. |
| [32] | Xing, Q.; Ma, Y.; Xie, H.; Xiao, F.; Zhang, F.; Deng, G. J. J. Org. Chem. 2019, 84, 1238. |
| [33] | Xu, Z.; Huang, H.; Chen, H.; Deng, G. J. Org. Chem. Front. 2019, 6, 3060. |
| [34] | Wang, X.; Qiu, X.; Wei, J.; Liu, J.; Song, S.; Wang, W.; Jiao, N. Org. Lett. 2018, 20, 2632. |
| [35] | Ni, P.; Tan, J.; Li, R.; Huang, H.; Zhang, F.; Deng, G. J. RSC Adv. 2020, 10, 3931. |
| [36] | Wu, M.; Jiang, Y.; An, Z.; Qi, Z.; Yan, R. Adv. Synth. Catal. 2018, 360, 4236. |
| [37] | Childers, K. K.; Haidle, A. M.; Machacek, M. R.; Rogers, J. P.; Romeo, E. Tetrahedron Lett, 2013, 54, 2506. |
| [38] | Ma, X.; Yu, X.; Huang, H.; Zhou, Y.; Song, Q. Org. Lett. 2020, 22, 5284. |
| [39] | Zhou, Z.; Liu, M.; Sun, S.; Yao, E.; Liu, S.; Wu, Z.; Yu, J. T.; Jiang, Y.; Cheng, J. Terahedron Lett. 2017, 58, 2571. |
| [40] | Wang, Z.; Xie, H.; Xiao, F.; Guo, Y.; Huang, H.; Deng, G. J. Eur. J. Org. Chem. 2017, 2017, 1604. |
| [41] | Xie, H.; Cai, J.; Wang, Z.; Huang, H.; Deng, G. J. Org. Lett. 2016, 18, 2196. |
| [42] | Hagen, H.; Kohler, R. D.; Liebigs, H. F. Ann. Chem. 1980, 1216. |
| [43] | (a) Zhou, Z.; Liu, Y.; Chen, J.; Yao, E.; Cheng, J. Org. Lett. 2016, 18, 5268. |
| [43] | (b) Chen, J.; Jiang, Y.; Yu, J. T.; Cheng, J. J. Org. Chem. 2016, 81, 271. |
| [44] | Li, L.; Chen, Q.; Xu, H.; Zhang, X. H.; Zhang, X. G. J. Org. Chem. 2020, 85, 10083. |
| [45] | Xie, H.; Li, G.; Zhang, F.; Xiao, F.; Deng, G. J. Green Chem. 2018, 20, 827. |
| [46] | Abdelwahab, A. B.; Hanna, A. G.; Kirsch, G. Synthesis 2016, 48, 2881. |
| [47] | Huang, X. G.; Liu, J.; Ren, J.; Wang, T.; Chen, W.; Zeng, B. B. Tetrahedron, 2011, 67, 6202. |
| [48] | Akbarzadeh, A.; Dekamin, M. G. Green Chem. Lett. Rev. 2017, 10, 315. |
| [49] | Treu, M.; Karner, T.; Kousek, R.; Berger, H.; Mayer, M.; McConnell, D. B.; Stadler, A. J. Comb. Chem. 2008, 10, 863. |
| [50] | Tayebee, R.; Ahmadi, S. J.; Seresht, E. R.; Javadi, F.; Yasemi, M. A.; Hosseinpour, M.; Maleki, B. Ind. Eng. Chem. Res. 2012, 51, 14577. |
| [51] | Bai, R.; Liu, P.; Yang, J.; Liu, C.; Gu, Y. ACS Sustainable Chem. Eng. 2015, 3, 1292. |
| [52] | Wang, Z.; Qu, Z.; Xiao, F.; Huang, H.; Deng, G. J. Adv. Synth. Catal. 2018, 360, 796. |
| [53] | Nguyen, T. T. T.; Le, V A. Adv. Synth. Catal. 2019, 362, 160. |
| [54] | Nguyen, T. B.; Retailleau, P. Green Chem. 2018, 20, 387. |
| [55] | Chithiravel, R.; Rajaguru, K.; Muthusubramanian, S.; Bhuvanesh, N. RSC Adv. 2015, 5, 86414. |
| [56] | Han, Y.; Tang, W. Q.; Yan, C. Tetrahedron Lett. 2014, 55, 1441. |
| [57] | Zhang, G.; Yi, H.; Chen, H.; Bian, C.; Liu, C.; Lei, A. Org. Lett. 2014, 16, 6156. |
| [58] | (a) Kasano, Y.; Okada, A.; Hiratsuka, D.; Oderaotoshi, Y.; Minakata, S.; Komatsu, M. Tetrahedron 2006, 62, 537. |
| [58] | (b) Yang, S.CN 10654449, 2010. |
| [59] | Jin, S.; Kuang, Z.; Song, Q. Org. Lett. 2020, 22, 615. |
| [60] | Solovyev, A. Y.; Androsov, D. A.; Neckers, D. C. J. Org. Chem. 2007, 72, 3122. |
| [61] | Nguyen, T, B.; Retailleau, P. Org. Lett. 2017, 19, 4858. |
| [62] | Jiang, P.; Che, X.; Liao, Y.; Huang, H.; Deng, G. J. RSC Adv. 2016, 47, 41751. |
| [63] | (a) Higashino, T.; Ueda, A.; Yoshida, J.; Mori, H. Chem. Commun. 2017, 53, 3426. |
| [63] | (b) Hanekamp, J. C.; Klusener, P.; Brandsma, L. Synth. Commun 1989, 19, 2691. |
| [64] | Nagahora, N.; Takemoto, I.; Fujii, M.; Shioji, K.; Okuma, K. Org. Lett. 2017, 19, 2110. |
| [65] | Moon, S.; Kato, M.; Nishii, Y.; Miura, M. Adv. Synth. Catal. 2020, 362, 1669. |
| [66] | (a) Sakai, S.; Sato, K.; Yoshida, K. Tetrahedron Lett. 2020, 61, 151476. |
| [66] | (b) Meng, L.; Fujikawa, T.; Kuwayama, M.; Segawa, Y.; Itami, K. J. Am. Chem. Soc. 2016, 138, 10351. |
| [66] | (c) Takimiya, K.; Konda, Y.; Ebata, H.; Niihara, N.; Otsubo, T. J. Org. Chem. 2005, 70, 10569. |
| [66] | (d) Fedenok, L. G.; Fedotov, K. Y.; Pritchina, E. A.; Polyakov, N. E. Tetrahedron Lett. 2016, 57, 1273. |
| [66] | (e) Shoji, T.; Miura, K.; Ohta, A.; Sekiguchi, R.; Ito, S.; Endo, Y.; Nagahata, T.; Mori, S.; Okujima, T. Org. Chem. Front. 2019, 6, 2801. |
| [67] | Huang, H.; Xu, Z.; Ji, X.; Li, B.; Deng, G. J. Org. Lett. 2018, 20, 4917. |
| [68] | Zhou, P.; Huang, Y.; Wu, W.; Yu, W.; Li, J.; Zhu, Z.; Jiang, H. Org. Biomol. Chem. 2019, 17, 3424. |
| [69] | Huang, H.; Wang, Q.; Xu, Z.; Deng, G. J. Adv. Synth. Catal. 2019, 361, 591. |
| [70] | Pham, P. H.; Nguyen, K. X.; Pham, H. T. B.; Tran, T. T.; Nguyen, T. T.; Phan, N. T. S. RSC Adv. 2020, 10, 11024. |
| [71] | Huang, H.; Qu, Z.; Ji, X.; Deng, G. J. Org. Chem. Front. 2019, 6, 1146. |
| [72] | Zhou, P.; Huang, Y.; Wu, W.; Zhou, J.; Yu, W.; Jiang, H. Org. Lett. 2019, 21, 9976. |
| [73] | Zhang, W.; Tao, S.; Ge, H.; Li, Q.; Ai, Z.; Li, X.; Zhang, B.; Sun, F.; Xu, X.; Du, Y. Org. Lett. 2020, 22, 448. |
| [74] | Pham, P. H.; Nguyen, K. X.; Pham, H. T. B.; Nguyen, T. T.; Phan, N. T. S. Org. Lett. 2019, 21, 8795. |
| [75] | Yue, Y.; Shao, H.; Wang, Z.; Wang, K.; Wang, L.; Zhuo, K.; Liu, J. J. Org. Chem. 2020, 85, 11265. |
| [76] | Liu, J.; Zhang, Y.; Yue, Y.; Wang, Z.; Shao, H.; Zhuo, K.; Lv, Q.; Zhang, Z. J. Org. Chem. 2019, 84, 12946. |
| [77] | Li, B.; Ni, P.; Huang, H.; Xiao, F.; Deng, G. J. Adv. Synth. Catal. 2017, 359, 4300. |
| [78] | Ni, P.; Li, B.; Huang, H.; Xiao, F.; Deng, G. J. Green Chem. 2017, 19, 5553. |
| [79] | Liao, Y.; Peng, Y.; Qi, H.; Deng, G. J.; Gong, H.; Li, C. Chem. Commun. 2015, 51, 1031. |
| [80] | Lu, C.; Huang, H.; Tuo, X.; Jiang, P.; Zhang, F.; Deng, G. J. Org. Chem. Front. 2019, 6, 2738. |
| [81] | Chitrala, T.; Rahman, N. K. F. Org Lett, 2020, 22, 1726. |
| [82] | Xu, Z.; Deng, G. J.; Zhang, F.; Chen, H.; Huang, H. Org. Lett. 2019, 21, 8630. |
| [83] | Chen, J.; Li, G.; Xie, Y.; Liao, Y.; Xiao, F.; Deng, G. J. Org. Lett., 2015, 17, 5870. |
| [84] | Jiang, J.; Tuo, X.; Fu, Z.; Huang, H.; Deng, G. J. Org. Biomol. Chem. 2020, 18, 3234. |
| [85] | Nguyen, T. B.; Retailleau, P.; Org. Lett. 2018, 20, 186. |
| [86] | Chen, F. J.; Liao, G.; Li, X.; Wu, J. Shi, B. F. Org. Lett. 2014, 16, 5644. |
| [87] | Zhang, B.; Liu, D.; Sun, Y.; Zhang, Y.; Feng, J.; Yu, F. Org. Lett. 2021, 23, 8, 3076. |
/
| 〈 |
|
〉 |