有机化学 ›› 2022, Vol. 42 ›› Issue (4): 1002-1012.DOI: 10.6023/cjoc202109038 上一篇 下一篇
综述与进展
收稿日期:2021-09-24
发布日期:2021-12-08
通讯作者:
肖立伟
基金资助:
Liwei Xiao( ), Guangxian Liu, Ping Ren, Tongtong Wu, Yuwei Lu, Jie Kong
), Guangxian Liu, Ping Ren, Tongtong Wu, Yuwei Lu, Jie Kong
Received:2021-09-24
Published:2021-12-08
Contact:
Liwei Xiao
Supported by:文章分享
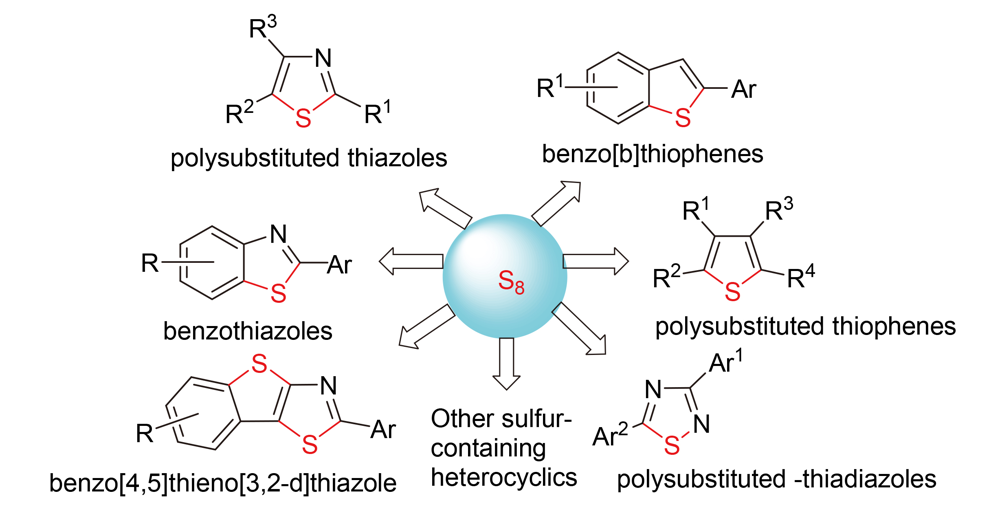
含硫杂环化合物在药物、分子器件和材料等诸多领域应用广泛, 因而其合成方法备受关注. 合成含硫杂环的关键在于分子中C—S键的构建, 一般通过含硫有机物或无机物参与的C—H键功能化或C—X键偶联反应来实现. 单质硫价廉易得, 性质稳定, 是合成含硫杂环的优质硫原子供体, 因而以单质硫为原料合成含硫杂环成为人们研究的热点. 综述了近年来以单质硫为硫源合成各种含硫杂环的研究进展情况.
肖立伟, 刘光仙, 任萍, 吴彤桐, 卢玉伟, 孔洁. 单质硫: 合成含硫杂环的优质硫源[J]. 有机化学, 2022, 42(4): 1002-1012.
Liwei Xiao, Guangxian Liu, Ping Ren, Tongtong Wu, Yuwei Lu, Jie Kong. Elemental Sulfur: An Excellent Sulfur-Source for Synthesis of Sulfur-Containing Heterocyclics[J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1002-1012.
| Entry | R2-FG | Product | Cat./base | Reaction condition | Yield/% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Aldehyde | 2-Arylbenzothiazole | CuCl2-Phen/K2CO3 | H2O, 100 ℃ | 52~96 | [ |
| 2 | Benzylchloride | 2-Arylbenzothiazole | Cu(OAc)2/Na2CO3 | DMSO, 130 ℃ | 53~98 | [ |
| 3 | Benzylamine | 2-Arylbenzothiazole | Cu(OAc)2/DABCO | DMSO, 100 ℃ | 10~90 | [ |
| 4 | Benzylamine | 2-Arylbenzothiazole | CuCl-Phen | H2O, 100 ℃ | 60~88 | [ |
| 5 | Terminalalkyne | 2-Benzylbenzothiazole | CuTC/DBU | MeCN/H2O, 130 ℃ | 45~84 | [ |
| 6 | Terminalalkyne | 2-Arylbenzothiazole | CuI-Phen/K2CO3 | DMSO, 110 ℃ | 62~90 | [ |
| 7 | N-Tosylhydrazone | 2-Arylbenzothiazole | CuSCN/DBU | DMSO, 110 ℃ | 63~84 | [ |
| 8 | N-Tosylhydrazone | 2-Benzylbenzothiazole | CuI, CsF/DABCO | MeCN, 130 ℃ | 57~70 | [ |
| 9 | Quaternaryammonium | 2-Alkylbenzothiazole | KOH | H2O, 140 ℃ | 74~95 | [ |
| Entry | R2-FG | Product | Cat./base | Reaction condition | Yield/% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Aldehyde | 2-Arylbenzothiazole | CuCl2-Phen/K2CO3 | H2O, 100 ℃ | 52~96 | [ |
| 2 | Benzylchloride | 2-Arylbenzothiazole | Cu(OAc)2/Na2CO3 | DMSO, 130 ℃ | 53~98 | [ |
| 3 | Benzylamine | 2-Arylbenzothiazole | Cu(OAc)2/DABCO | DMSO, 100 ℃ | 10~90 | [ |
| 4 | Benzylamine | 2-Arylbenzothiazole | CuCl-Phen | H2O, 100 ℃ | 60~88 | [ |
| 5 | Terminalalkyne | 2-Benzylbenzothiazole | CuTC/DBU | MeCN/H2O, 130 ℃ | 45~84 | [ |
| 6 | Terminalalkyne | 2-Arylbenzothiazole | CuI-Phen/K2CO3 | DMSO, 110 ℃ | 62~90 | [ |
| 7 | N-Tosylhydrazone | 2-Arylbenzothiazole | CuSCN/DBU | DMSO, 110 ℃ | 63~84 | [ |
| 8 | N-Tosylhydrazone | 2-Benzylbenzothiazole | CuI, CsF/DABCO | MeCN, 130 ℃ | 57~70 | [ |
| 9 | Quaternaryammonium | 2-Alkylbenzothiazole | KOH | H2O, 140 ℃ | 74~95 | [ |
| Entry | Ar-FG | Product | Cat. | Reaction condition | Yield/% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Methylaromatic | 2-Arylbenzothiazole | — | 275 ℃ | 15~74 | [ |
| 2 | Methylaromatic | 2-Arylnaphthothiazole | — | 275 ℃ | 41~50 | [ |
| 3 | 2-Methylarene | 2-Heteroarylbenzothiazole | NH4I | NMP, air, 160 ℃ | 37~87 | [ |
| 4 | Arylaldehyde | 2-Arylnaphtho[2,1-d]thiazole | NH4I/4 Å MS | DMSO/PhCl, 140 ℃ | 61~98 | [ |
| 5 | Arylaldehyde | 2-Arylbenzothiazole | KI | O2, NMP, 150 ℃ | 40~79 | [ |
| 6 | Styrene | 2-Benzylbenzothiazole | NH4I | NMP, air, 140 ℃ | 63~88 | [ |
| 7 | Arylacetylene | 2-Benzylbenzothiazole | NH4I | NMP, air, 140 ℃ | 57~81 | [ |
| 8 | Ether | 2-Aryl, alkylbenzothiazole | TBHPb/KI | DMSO, air, 130 ℃ | 41~81 | [ |
| 9 | Aliphaticamine | 2-Aryl, alkylbenzothiazole | sealed tube | DMSO, 140 ℃ | 42~90 | [ |
| 10 | Acetophenone | 2-Arylbenzothiazole | I2, TBAIc | DMSO/PhCl, 140 ℃ | 40~81 | [ |
| 11 | Acetophenone | 2-Aroylbenzothiazole | I2, | DMSO/PhCl, 140 ℃ | 42~78 | [ |
| 12 | Isatin | 2-(2'-Aminophenyl)benzothiaole | KI/4 Å MS | DMSO, 120 ℃ | 60~85 | [ |
| Entry | Ar-FG | Product | Cat. | Reaction condition | Yield/% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Methylaromatic | 2-Arylbenzothiazole | — | 275 ℃ | 15~74 | [ |
| 2 | Methylaromatic | 2-Arylnaphthothiazole | — | 275 ℃ | 41~50 | [ |
| 3 | 2-Methylarene | 2-Heteroarylbenzothiazole | NH4I | NMP, air, 160 ℃ | 37~87 | [ |
| 4 | Arylaldehyde | 2-Arylnaphtho[2,1-d]thiazole | NH4I/4 Å MS | DMSO/PhCl, 140 ℃ | 61~98 | [ |
| 5 | Arylaldehyde | 2-Arylbenzothiazole | KI | O2, NMP, 150 ℃ | 40~79 | [ |
| 6 | Styrene | 2-Benzylbenzothiazole | NH4I | NMP, air, 140 ℃ | 63~88 | [ |
| 7 | Arylacetylene | 2-Benzylbenzothiazole | NH4I | NMP, air, 140 ℃ | 57~81 | [ |
| 8 | Ether | 2-Aryl, alkylbenzothiazole | TBHPb/KI | DMSO, air, 130 ℃ | 41~81 | [ |
| 9 | Aliphaticamine | 2-Aryl, alkylbenzothiazole | sealed tube | DMSO, 140 ℃ | 42~90 | [ |
| 10 | Acetophenone | 2-Arylbenzothiazole | I2, TBAIc | DMSO/PhCl, 140 ℃ | 40~81 | [ |
| 11 | Acetophenone | 2-Aroylbenzothiazole | I2, | DMSO/PhCl, 140 ℃ | 42~78 | [ |
| 12 | Isatin | 2-(2'-Aminophenyl)benzothiaole | KI/4 Å MS | DMSO, 120 ℃ | 60~85 | [ |
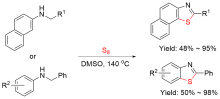
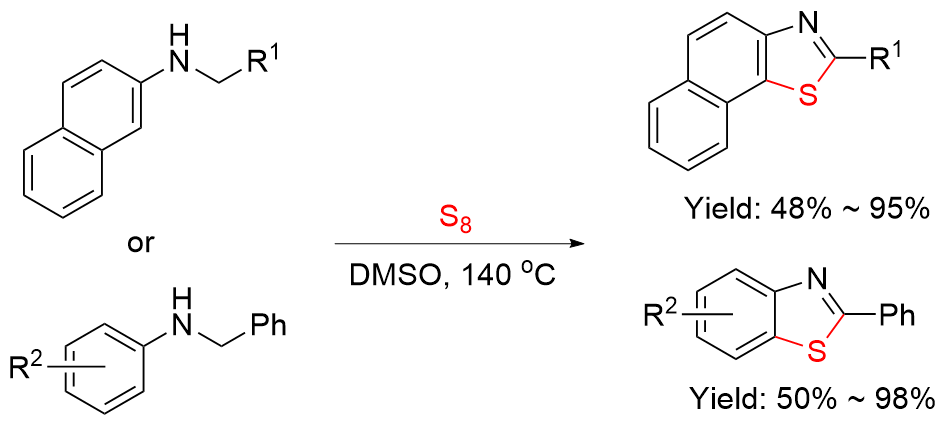
| Entry | Ar-FG | Product | Cat./base | Reaction condition | Yield% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Methylhetarene | 2-Hetarylbenzothiazole | — | 120 ℃ | 37~85 | [ |
| 2 | Methylhetarene | 2-Hetarylbenzothiazole | NH4I | Sulfolane, 175 ℃ | 9~60 | [ |
| 3 | Benzaldehyde | 2-Arylbenzothiazole | NMM | 130 ℃ | 60~80 | [ |
| 4 | Benzylamine | 2-Arylbenzothiazole | Pyridine | 100 ℃ | 61~80 | [ |
| 5 | Aliphatic amine | 2-Arylbenzothiazole | — | 130 ℃ | 52~80 | [ |
| 6 | Acetophenone | 2-Aroylbenzothiazole | NMM | 120 ℃ | 52~92 | [ |
| 7 | Arylacetic acid | 2-Arylbenzothiazole | NMM | 110 ℃ | 46~75 | [ |
| 8 | Benzylic alcohol | 2-Arylbenzothiazole | FeCl3/KHCO3/NH4I | NMP, 160 ℃ | 25~80 | [ |
| Entry | Ar-FG | Product | Cat./base | Reaction condition | Yield% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Methylhetarene | 2-Hetarylbenzothiazole | — | 120 ℃ | 37~85 | [ |
| 2 | Methylhetarene | 2-Hetarylbenzothiazole | NH4I | Sulfolane, 175 ℃ | 9~60 | [ |
| 3 | Benzaldehyde | 2-Arylbenzothiazole | NMM | 130 ℃ | 60~80 | [ |
| 4 | Benzylamine | 2-Arylbenzothiazole | Pyridine | 100 ℃ | 61~80 | [ |
| 5 | Aliphatic amine | 2-Arylbenzothiazole | — | 130 ℃ | 52~80 | [ |
| 6 | Acetophenone | 2-Aroylbenzothiazole | NMM | 120 ℃ | 52~92 | [ |
| 7 | Arylacetic acid | 2-Arylbenzothiazole | NMM | 110 ℃ | 46~75 | [ |
| 8 | Benzylic alcohol | 2-Arylbenzothiazole | FeCl3/KHCO3/NH4I | NMP, 160 ℃ | 25~80 | [ |
| Entry | Ar2-FG | Product | Cat./base | Reaction condition | Yield/% | Ref.. |
|---|---|---|---|---|---|---|
| 1 | Arylmethylbromide | 3,5-Diaryl-1,2,4-thiadiazole | LiOtBu | Toluene, 140 ℃ | 45~67 | [ |
| 2 | Methylketone | 3-Aryl-5-acyl-1,2,4-thiadiazole | PaCl2/K2HPO4 | DMSO, 130 ℃ | 35~75 | [ |
| 3 | 2-Methylquinoline | 3,5-Diaryl-1,2,4-thiadiazole | K3PO4 | DMSO, 120 ℃ | 42~93 | [ |
| 4 | Aromaticaldehyde | 3,5-Diaryl-1,2,4-thiadiazole | K3PO4 | DMSO, 120 ℃ | 48~63 | [ |
| Entry | Ar2-FG | Product | Cat./base | Reaction condition | Yield/% | Ref.. |
|---|---|---|---|---|---|---|
| 1 | Arylmethylbromide | 3,5-Diaryl-1,2,4-thiadiazole | LiOtBu | Toluene, 140 ℃ | 45~67 | [ |
| 2 | Methylketone | 3-Aryl-5-acyl-1,2,4-thiadiazole | PaCl2/K2HPO4 | DMSO, 130 ℃ | 35~75 | [ |
| 3 | 2-Methylquinoline | 3,5-Diaryl-1,2,4-thiadiazole | K3PO4 | DMSO, 120 ℃ | 42~93 | [ |
| 4 | Aromaticaldehyde | 3,5-Diaryl-1,2,4-thiadiazole | K3PO4 | DMSO, 120 ℃ | 48~63 | [ |
| Entry | Substrate 1 | Substrate 2 | Cat./base | Reaction condition | Yield/% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Ketone | Cyanoacetone | Piperidine | EtOH, 55~65 ℃ | 8~86 | [ |
| 2 | Ketone, aldehyde | Dicyanomethane | Imidazole | DMF, 60 ℃, N2 | 27~88 | [ |
| 3 | Ketone, aldehyde | Cyanomethylene | Morpholine | PEG-600, U.S. | 29~98 | [ |
| 4 | Cycloketone | Ethylcyanoacetate | Morpholine | M.W., 120 ℃ | 22~87 | [ |
| 5 | Ketone, aldehyde | Malononitrile | ZnO | 100 ℃ | 37~86 | [ |
| 6 | Ketone | Malononitrile | NaAlO2 | EtOH, 60 ℃ | 26~97 | [ |
| 7 | Arylacetaldehyde | 1,3-Diketone | K2CO3/KHCO3 | DMSO, 95 ℃ | 36~84 | [ |
| 8 | Chalcone | Arylacetonitrile | DBU | DMSO/DABCO, 80 ℃ | 43~75 | [ |
| 9 | Arylmethylketone | Arylmethylketone | Anline/p-TsA | 120 ℃, Ar | 48~88 | [ |
| Entry | Substrate 1 | Substrate 2 | Cat./base | Reaction condition | Yield/% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Ketone | Cyanoacetone | Piperidine | EtOH, 55~65 ℃ | 8~86 | [ |
| 2 | Ketone, aldehyde | Dicyanomethane | Imidazole | DMF, 60 ℃, N2 | 27~88 | [ |
| 3 | Ketone, aldehyde | Cyanomethylene | Morpholine | PEG-600, U.S. | 29~98 | [ |
| 4 | Cycloketone | Ethylcyanoacetate | Morpholine | M.W., 120 ℃ | 22~87 | [ |
| 5 | Ketone, aldehyde | Malononitrile | ZnO | 100 ℃ | 37~86 | [ |
| 6 | Ketone | Malononitrile | NaAlO2 | EtOH, 60 ℃ | 26~97 | [ |
| 7 | Arylacetaldehyde | 1,3-Diketone | K2CO3/KHCO3 | DMSO, 95 ℃ | 36~84 | [ |
| 8 | Chalcone | Arylacetonitrile | DBU | DMSO/DABCO, 80 ℃ | 43~75 | [ |
| 9 | Arylmethylketone | Arylmethylketone | Anline/p-TsA | 120 ℃, Ar | 48~88 | [ |
| Entry | R2-FG | Product | Cat./base | Reaction condition | Yield% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Arylaldehyde | 2-Arylbenzothienothiazole | CuBr/Li2CO3 | DMSO, 120 ℃ | 48~91 | [ |
| 2 | Arylacetylene | 2-Arylbenzothienothiazoleb | CuOTf/TBD | DMSO/hexane, 120 ℃ | 44~83 | [ |
| 3 | Arylaldehyde | 2-Arylbenzothienothiazoleb | CuCl/TBD | DMSO/hexane, 120 ℃ | 41~90 | [ |
| 4 | Methyl-N-heteroarene | 2-Arylbenzothienothiazole | Cs2CO3 | DMSO, 120 ℃ | 22~62 | [ |
| 5 | Arylacetic acid or ester | 2-Arylbenzothienothiazole | Li2CO3 | DMSO, 120 ℃ | 65~92 | [ |
| 6 | Isothiocyanate | 2-Aminobenzothienothiazole | KSCN/Li2CO3 | DMSO, 120 ℃ | 32~82 | [ |
| 7 | Acetophenone | 2-Aroylthienothiazole | TBD | DMSO, 120 ℃ | 31~87 | [ |
| Entry | R2-FG | Product | Cat./base | Reaction condition | Yield% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Arylaldehyde | 2-Arylbenzothienothiazole | CuBr/Li2CO3 | DMSO, 120 ℃ | 48~91 | [ |
| 2 | Arylacetylene | 2-Arylbenzothienothiazoleb | CuOTf/TBD | DMSO/hexane, 120 ℃ | 44~83 | [ |
| 3 | Arylaldehyde | 2-Arylbenzothienothiazoleb | CuCl/TBD | DMSO/hexane, 120 ℃ | 41~90 | [ |
| 4 | Methyl-N-heteroarene | 2-Arylbenzothienothiazole | Cs2CO3 | DMSO, 120 ℃ | 22~62 | [ |
| 5 | Arylacetic acid or ester | 2-Arylbenzothienothiazole | Li2CO3 | DMSO, 120 ℃ | 65~92 | [ |
| 6 | Isothiocyanate | 2-Aminobenzothienothiazole | KSCN/Li2CO3 | DMSO, 120 ℃ | 32~82 | [ |
| 7 | Acetophenone | 2-Aroylthienothiazole | TBD | DMSO, 120 ℃ | 31~87 | [ |
| Entry | R2-FG | Product | Cat./acid | Reaction condition | Yield% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Alkyne | Thieno[2,3-b]indole | HCl | DMF, 150 ℃ | 31~82 | [ |
| 2 | Alkene | Thieno[2,3-b]indole | HCl/HOAc | DMF, 150 ℃ | 30~78 | [ |
| 4 | Acetophenone | 3-Aryl thieno[2,3-b]indole | HI/phenylalanine | CF3Ph/dioxane, 130 ℃ | 28~71 | [ |
| 5 | Acetophenone | 2-Aryl thieno[2,3-b]indole | HOAc | DMF, 150 ℃ | 37~84 | [ |
| 3 | Cyclohexanone | Benzothieno[2,3-b]indole | PdI2/CPDO a | o-Cl2C6H4, 125 ℃ | 38~72 | [ |
| 6 | Cyclohexanone | Benzothieno[2,3-b]indole | I2 | NMPc, 150 ℃ | 28~83 | [ |
| 7 | Cyclohexanone | 3-Arylindole | BF3/DPE b | DMF, 150 ℃ | 33~89 | [ |
| 8 | Cyclohexanone | 3-Arylindole | IBr | DPE/DMAd, 150 ℃ | 60~93 | [ |
| Entry | R2-FG | Product | Cat./acid | Reaction condition | Yield% | Ref. |
|---|---|---|---|---|---|---|
| 1 | Alkyne | Thieno[2,3-b]indole | HCl | DMF, 150 ℃ | 31~82 | [ |
| 2 | Alkene | Thieno[2,3-b]indole | HCl/HOAc | DMF, 150 ℃ | 30~78 | [ |
| 4 | Acetophenone | 3-Aryl thieno[2,3-b]indole | HI/phenylalanine | CF3Ph/dioxane, 130 ℃ | 28~71 | [ |
| 5 | Acetophenone | 2-Aryl thieno[2,3-b]indole | HOAc | DMF, 150 ℃ | 37~84 | [ |
| 3 | Cyclohexanone | Benzothieno[2,3-b]indole | PdI2/CPDO a | o-Cl2C6H4, 125 ℃ | 38~72 | [ |
| 6 | Cyclohexanone | Benzothieno[2,3-b]indole | I2 | NMPc, 150 ℃ | 28~83 | [ |
| 7 | Cyclohexanone | 3-Arylindole | BF3/DPE b | DMF, 150 ℃ | 33~89 | [ |
| 8 | Cyclohexanone | 3-Arylindole | IBr | DPE/DMAd, 150 ℃ | 60~93 | [ |
| [1] |
(a) Cui, S. F.; Wan, Y.; Lv, J. S.; Damu, G. L. V.; Zhou, C. H. Sci. China Chem. 2012, 42, 1105. (in Chinese)
|
|
( 崔胜峰, 王艳, 吕敬松, Damu, Guri, L. V.; 周成合; 中国科学: 化学, 2012, 42, 1105.)
|
|
|
(b) He, W.; Liu, D.; Gan, X.; Zhang, J.; Liu, Z.; Yi, C.; Song, B. Chin. J. Org. Chem. 2019, 39, 2287. (in Chinese)
|
|
|
( 何文静, 刘登曰, 甘秀海, 张建, 刘峥军, 易崇粉, 宋宝安, 有机化学. 2019, 39, 2287.)
|
|
| [2] |
(a) Xiao, L. W.; Xiao, S. Q.; Li, Z.; Jing, X. M.; Dai, F. C. Chemistry 2019, 82, 120. (in Chinese)
|
|
( 肖立伟, 肖树强, 李政, 景学敏, 戴富才, 化学通报, 2019, 82, 120.)
|
|
|
(b) Xiao, L. W.; Liu, G. X.; Jing, X. M.;Ren L. L.; Xiao, S. Q. Chem. Res. Appl. 2020, 32, 297. (in Chinese)
|
|
|
( 肖立伟, 刘光仙, 景学敏, 任丽磊, 肖树强, 化学研究与应用, 2020, 32, 297.)
|
|
| [3] |
(a) Beletskaya, I. P.; Ananikov, V. P. Chem. Rev. 2011, 111, 1596.
doi: 10.1021/cr100347k pmid: 21391564 |
|
(b) Yang, W.; Zhang, M.; Chen, W.; Yang, X.; Feng, J. Chin. J. Org. Chem. 2020, 40, 4060. (in Chinese)
doi: 10.6023/cjoc202005039 pmid: 21391564 |
|
|
( 杨文超, 张明明, 陈旺, 杨小虎, 冯建国, 有机化学, 2020, 40, 4060.)
pmid: 21391564 |
|
| [4] |
(a) Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T. S. A.; Liu, X. Chem. Soc. Rev. 2015, 44, 291.
doi: 10.1039/C4CS00239C pmid: 33528480 |
|
(b) Sundaravelu, N.; Sangeetha, S.; Sekar, G. Org. Biomol. Chem. 2021, 19, 1459.
doi: 10.1039/d0ob02320e pmid: 33528480 |
|
| [5] |
(a) Nguyena, T. B. Adv. Synth. Catal. 2017, 359, 1066.
doi: 10.1002/adsc.201601329 |
|
(b) Li, J.; Yang, S.; Wu, W.; Jiang, H. Org. Chem. Front. 2020, 7, 1395.
doi: 10.1039/D0QO00377H |
|
|
(c) Nguyen, T. B. Adv. Synth. Catal. 2020, 362, 3448.
doi: 10.1002/adsc.202000535 |
|
|
(d) Fan, R.; Tan, C.; Liu, Y.; Wei, Y.; Zhao, X.; Liu, X.; Tan, J.; Yoshida, H. Chin. Chem. Lett. 2021, 32, 299.
doi: 10.1016/j.cclet.2020.06.003 |
|
|
(e) Cheng, H.; Guo, P.; Chen, B.; Yao, J.; Ma, J.; Hu, W.; Ji, H. Chin. J. Org. Chem. 2021, 41, 94. (in Chinese)
doi: 10.6023/cjoc202006032 |
|
|
( 程辉成, 郭鹏虎, 陈冰, 姚嘉伟, 马姣丽, 胡炜杰, 纪红兵, 有机化学, 2021, 41, 94.)
|
|
| [6] |
(a) Colebourne, N.; Foster, R. G.; Robson, E. J. Chem. Soc. C 1967, 685.
|
|
(b) Beck, G.; Heitzer, H.; Holtschmidt, H. Synthesis 1985, 586.
|
|
|
(c) Gewald, V. K.; Böttcher, H.; Mayer, R. J. Prakt. Chem. 1964, 23, 298.
|
|
|
(d) Gewald, V. K.; Spies, H.; Mayer, R. J. Prakt. Chem. 1970, 312, 776.
doi: 10.1002/prac.19703120507 |
|
| [7] |
Deng, H.; Li, Z.; Ke, F.; Zhou, X. Chem.-Eur. J. 2012, 18, 4840.
doi: 10.1002/chem.201103525 |
| [8] |
Yang, Z.; Hu, R.; Li, X.; Wang, X.; Gu, R.; Han, S. Tetrahedron Lett. 2017, 58, 2366.
doi: 10.1016/j.tetlet.2017.05.004 |
| [9] |
Wang, R.; Ding, Y. l.; Liu, H.; Peng, S.; Ren, J.; Li, L. Tetrahedron Lett. 2014, 55, 945.
doi: 10.1016/j.tetlet.2013.12.054 |
| [10] |
Xu, H.; Luo, C.; Li, Z.; Xiang, H.; Zhou, X. J. Heterocycl. Chem. 2015, 53, 120.
|
| [11] |
Huang, Y.; Yan, D.; Wang, X.; Zhou, P.; Wu, W.; Jiang, H. Chem. Commun. 2018, 54, 1742.
doi: 10.1039/C7CC09855C |
| [12] |
Huang, Y.; Zhou, P.; Wu, W.; Jiang, H. J. Org. Chem. 2018, 83, 2460.
doi: 10.1021/acs.joc.7b03118 |
| [13] |
Pan, L.; Yu, L.; Wu, Z.; Li, Z.; Xiang, H.; Zhou, X. RSC Adv. 2015, 46, 27775.
|
| [14] |
Zhang, L. F.; Ni, Z. H.; Li, D. Y.; Qin, Z. H.; Wei, X. Y. Chin. Chem. Lett. 2012, 23, 281.
doi: 10.1016/j.cclet.2011.12.004 |
| [15] |
Li, G.; Xie, H.; Chen, J.; Guo, Y.; Deng, G. J. Green Chem. 2017, 19, 4043.
doi: 10.1039/C7GC01932G |
| [16] |
Che, X.; Jiang, J.; Xiao, F.; Huang, H.; Deng, G. J. Org. Lett. 2017, 19, 4576.
doi: 10.1021/acs.orglett.7b02168 |
| [17] |
Deng, G.; Jiang, J.; Li, G.; Zhang, F.; Xie, H. Adv. Synth. Catal. 2018, 360, 1622.
doi: 10.1002/adsc.201701560 |
| [18] |
Zhang, J.; Zhao, X.; Liu, P.; Sun, P. J. Org. Chem. 2019, 84, 12596.
doi: 10.1021/acs.joc.9b02145 pmid: 31502839 |
| [19] |
Zhu, X.; Zhou, F.; Yang, Y.; Deng, G.; Liang, Y. ACS Omega 2020, 5, 22, 13136.
|
| [20] |
Liu, Y.; Yuan, X.; Guo, X.; Zhang, X.; Chen, B. Tetrahedron 2018, 74, 6057.
doi: 10.1016/j.tet.2018.08.047 |
| [21] |
Huynh, T. V.; Doan, K. V.; Luong, N. T. K.; Nguyen, D. T. P.; Doan, S. H.; Nguyen, T. T.; Phan, N. T. S. RSC Adv. 2020, 10, 18423.
doi: 10.1039/D0RA01750G |
| [22] |
Singh, M.; Vaishali, Paul, A. K.; Singh, V. Org. Biomol. Chem. 2020, 18, 4459.
doi: 10.1039/D0OB00888E |
| [23] |
Zhu, X.; Yang, Y.; Xiao, G.; Song, J.; Liang, Y.; Deng, G. Chem. Commun. 2017, 53, 11917.
doi: 10.1039/C7CC07366F |
| [24] |
Fu, R. G.; Wang, Y.; Xia, F.; Zhang, H. L.; Sun, Y.; Yang, D. W.; Wang, Y. W.; Yin, P. J. Org. Chem. 2019, 84, 12237.
doi: 10.1021/acs.joc.9b02032 |
| [25] |
Nguyen, T. B.; Ermolenko, L.; Al-Mourabit, A. Org. Lett. 2013, 15, 4218.
doi: 10.1021/ol401944a pmid: 23924277 |
| [26] |
Teramoto, M.; Imoto, M.; Takeda, M.; Mizuno, T.; Nomoto, A.; Ogawa, A. J. Org. Chem. 2020, 85, 15213.
doi: 10.1021/acs.joc.0c02072 |
| [27] |
Nguyen, L. A.; Ngo, Q. A.; Retailleau, P.; Nguyen, T. B. Green Chem. 2017, 19, 4289.
doi: 10.1039/C7GC01825H |
| [28] |
Nguyen, T. B.; Ermolenko, L.; Retailleau, P.; Al-Mourabit, A. Angew. Chem., Int. Ed. 2014, 53, 13808.
doi: 10.1002/anie.201408397 |
| [29] |
Tong, Y.; Pan, Q.; Jiang, Z.; Miao, D.; Shi, X.; Han, S. Tetrahedron Lett. 2014, 55, 5499.
doi: 10.1016/j.tetlet.2014.08.037 |
| [30] |
Nguyen, T. B.; Pasturaud, K.; Ermolenko, L.; Al-Mourabit, A. Org. Lett. 2015, 17, 2562.
doi: 10.1021/acs.orglett.5b01182 pmid: 25929738 |
| [31] |
Guntreddi, T.; Vanjari, R.; Singh, K. N. Org. Lett. 2015, 17, 976.
doi: 10.1021/acs.orglett.5b00079 |
| [32] |
Xing, Q.; Ma, Y.; Xie, H.; Xiao, F.; Zhang, F.; Deng, G. J. J. Org. Chem. 2019, 84, 1238.
doi: 10.1021/acs.joc.8b02619 |
| [33] |
Xu, Z.; Huang, H.; Chen, H.; Deng, G. J. Org. Chem. Front. 2019, 6, 3060.
doi: 10.1039/C9QO00592G |
| [34] |
Wang, X.; Qiu, X.; Wei, J.; Liu, J.; Song, S.; Wang, W.; Jiao, N. Org. Lett. 2018, 20, 2632.
doi: 10.1021/acs.orglett.8b00840 |
| [35] |
Ni, P.; Tan, J.; Li, R.; Huang, H.; Zhang, F.; Deng, G. J. RSC Adv. 2020, 10, 3931.
doi: 10.1039/C9RA09656F |
| [36] |
Wu, M.; Jiang, Y.; An, Z.; Qi, Z.; Yan, R. Adv. Synth. Catal. 2018, 360, 4236.
doi: 10.1002/adsc.201800693 |
| [37] |
Childers, K. K.; Haidle, A. M.; Machacek, M. R.; Rogers, J. P.; Romeo, E. Tetrahedron Lett, 2013, 54, 2506.
doi: 10.1016/j.tetlet.2013.03.014 |
| [38] |
Ma, X.; Yu, X.; Huang, H.; Zhou, Y.; Song, Q. Org. Lett. 2020, 22, 5284.
doi: 10.1021/acs.orglett.0c01275 |
| [39] |
Zhou, Z.; Liu, M.; Sun, S.; Yao, E.; Liu, S.; Wu, Z.; Yu, J. T.; Jiang, Y.; Cheng, J. Terahedron Lett. 2017, 58, 2571.
doi: 10.1016/j.tetlet.2017.05.061 |
| [40] |
Wang, Z.; Xie, H.; Xiao, F.; Guo, Y.; Huang, H.; Deng, G. J. Eur. J. Org. Chem. 2017, 2017, 1604.
doi: 10.1002/ejoc.201700148 |
| [41] |
Xie, H.; Cai, J.; Wang, Z.; Huang, H.; Deng, G. J. Org. Lett. 2016, 18, 2196.
doi: 10.1021/acs.orglett.6b00806 |
| [42] |
Hagen, H.; Kohler, R. D.; Liebigs, H. F. Ann. Chem. 1980, 1216.
|
| [43] |
(a) Zhou, Z.; Liu, Y.; Chen, J.; Yao, E.; Cheng, J. Org. Lett. 2016, 18, 5268.
doi: 10.1021/acs.orglett.6b02583 |
|
(b) Chen, J.; Jiang, Y.; Yu, J. T.; Cheng, J. J. Org. Chem. 2016, 81, 271.
doi: 10.1021/acs.joc.5b02280 |
|
| [44] |
Li, L.; Chen, Q.; Xu, H.; Zhang, X. H.; Zhang, X. G. J. Org. Chem. 2020, 85, 10083.
doi: 10.1021/acs.joc.0c01334 |
| [45] |
Xie, H.; Li, G.; Zhang, F.; Xiao, F.; Deng, G. J. Green Chem. 2018, 20, 827.
doi: 10.1039/C7GC03599C |
| [46] |
Abdelwahab, A. B.; Hanna, A. G.; Kirsch, G. Synthesis 2016, 48, 2881.
doi: 10.1055/s-0035-1561459 |
| [47] |
Huang, X. G.; Liu, J.; Ren, J.; Wang, T.; Chen, W.; Zeng, B. B. Tetrahedron, 2011, 67, 6202.
doi: 10.1016/j.tet.2011.06.061 |
| [48] |
Akbarzadeh, A.; Dekamin, M. G. Green Chem. Lett. Rev. 2017, 10, 315.
doi: 10.1080/17518253.2017.1380234 |
| [49] |
Treu, M.; Karner, T.; Kousek, R.; Berger, H.; Mayer, M.; McConnell, D. B.; Stadler, A. J. Comb. Chem. 2008, 10, 863.
doi: 10.1021/cc800081b |
| [50] |
Tayebee, R.; Ahmadi, S. J.; Seresht, E. R.; Javadi, F.; Yasemi, M. A.; Hosseinpour, M.; Maleki, B. Ind. Eng. Chem. Res. 2012, 51, 14577.
doi: 10.1021/ie301737h |
| [51] |
Bai, R.; Liu, P.; Yang, J.; Liu, C.; Gu, Y. ACS Sustainable Chem. Eng. 2015, 3, 1292.
doi: 10.1021/sc500763q |
| [52] |
Wang, Z.; Qu, Z.; Xiao, F.; Huang, H.; Deng, G. J. Adv. Synth. Catal. 2018, 360, 796.
doi: 10.1002/adsc.201701332 |
| [53] |
Nguyen, T. T. T.; Le, V A. Adv. Synth. Catal. 2019, 362, 160.
doi: 10.1002/adsc.201901235 |
| [54] |
Nguyen, T. B.; Retailleau, P. Green Chem. 2018, 20, 387.
doi: 10.1039/C7GC03437G |
| [55] |
Chithiravel, R.; Rajaguru, K.; Muthusubramanian, S.; Bhuvanesh, N. RSC Adv. 2015, 5, 86414.
doi: 10.1039/C5RA17829K |
| [56] |
Han, Y.; Tang, W. Q.; Yan, C. Tetrahedron Lett. 2014, 55, 1441.
|
| [57] |
Zhang, G.; Yi, H.; Chen, H.; Bian, C.; Liu, C.; Lei, A. Org. Lett. 2014, 16, 6156.
doi: 10.1021/ol503015b |
| [58] |
(a) Kasano, Y.; Okada, A.; Hiratsuka, D.; Oderaotoshi, Y.; Minakata, S.; Komatsu, M. Tetrahedron 2006, 62, 537.
doi: 10.1016/j.tet.2005.10.024 |
|
(b) Yang, S.CN 10654449, 2010.
|
|
| [59] |
Jin, S.; Kuang, Z.; Song, Q. Org. Lett. 2020, 22, 615.
doi: 10.1021/acs.orglett.9b04389 |
| [60] |
Solovyev, A. Y.; Androsov, D. A.; Neckers, D. C. J. Org. Chem. 2007, 72, 3122.
pmid: 17375954 |
| [61] |
Nguyen, T, B.; Retailleau, P. Org. Lett. 2017, 19, 4858.
doi: 10.1021/acs.orglett.7b02321 pmid: 28840729 |
| [62] |
Jiang, P.; Che, X.; Liao, Y.; Huang, H.; Deng, G. J. RSC Adv. 2016, 47, 41751.
|
| [63] |
(a) Higashino, T.; Ueda, A.; Yoshida, J.; Mori, H. Chem. Commun. 2017, 53, 3426.
doi: 10.1039/C7CC00784A |
|
(b) Hanekamp, J. C.; Klusener, P.; Brandsma, L. Synth. Commun 1989, 19, 2691.
doi: 10.1080/00397918908053063 |
|
| [64] |
Nagahora, N.; Takemoto, I.; Fujii, M.; Shioji, K.; Okuma, K. Org. Lett. 2017, 19, 2110.
doi: 10.1021/acs.orglett.7b00716 |
| [65] |
Moon, S.; Kato, M.; Nishii, Y.; Miura, M. Adv. Synth. Catal. 2020, 362, 1669.
doi: 10.1002/adsc.202000112 |
| [66] |
(a) Sakai, S.; Sato, K.; Yoshida, K. Tetrahedron Lett. 2020, 61, 151476.
doi: 10.1016/j.tetlet.2019.151476 pmid: 16323873 |
|
(b) Meng, L.; Fujikawa, T.; Kuwayama, M.; Segawa, Y.; Itami, K. J. Am. Chem. Soc. 2016, 138, 10351.
doi: 10.1021/jacs.6b06486 pmid: 16323873 |
|
|
(c) Takimiya, K.; Konda, Y.; Ebata, H.; Niihara, N.; Otsubo, T. J. Org. Chem. 2005, 70, 10569.
pmid: 16323873 |
|
|
(d) Fedenok, L. G.; Fedotov, K. Y.; Pritchina, E. A.; Polyakov, N. E. Tetrahedron Lett. 2016, 57, 1273.
doi: 10.1016/j.tetlet.2016.02.025 pmid: 16323873 |
|
|
(e) Shoji, T.; Miura, K.; Ohta, A.; Sekiguchi, R.; Ito, S.; Endo, Y.; Nagahata, T.; Mori, S.; Okujima, T. Org. Chem. Front. 2019, 6, 2801.
doi: 10.1039/c9qo00593e pmid: 16323873 |
|
| [67] |
Huang, H.; Xu, Z.; Ji, X.; Li, B.; Deng, G. J. Org. Lett. 2018, 20, 4917.
doi: 10.1021/acs.orglett.8b02049 |
| [68] |
Zhou, P.; Huang, Y.; Wu, W.; Yu, W.; Li, J.; Zhu, Z.; Jiang, H. Org. Biomol. Chem. 2019, 17, 3424.
doi: 10.1039/C9OB00377K |
| [69] |
Huang, H.; Wang, Q.; Xu, Z.; Deng, G. J. Adv. Synth. Catal. 2019, 361, 591.
doi: 10.1002/adsc.201801324 |
| [70] |
Pham, P. H.; Nguyen, K. X.; Pham, H. T. B.; Tran, T. T.; Nguyen, T. T.; Phan, N. T. S. RSC Adv. 2020, 10, 11024.
doi: 10.1039/D0RA00808G |
| [71] |
Huang, H.; Qu, Z.; Ji, X.; Deng, G. J. Org. Chem. Front. 2019, 6, 1146.
|
| [72] |
Zhou, P.; Huang, Y.; Wu, W.; Zhou, J.; Yu, W.; Jiang, H. Org. Lett. 2019, 21, 9976.
doi: 10.1021/acs.orglett.9b03900 |
| [73] |
Zhang, W.; Tao, S.; Ge, H.; Li, Q.; Ai, Z.; Li, X.; Zhang, B.; Sun, F.; Xu, X.; Du, Y. Org. Lett. 2020, 22, 448.
doi: 10.1021/acs.orglett.9b04206 pmid: 31894988 |
| [74] |
Pham, P. H.; Nguyen, K. X.; Pham, H. T. B.; Nguyen, T. T.; Phan, N. T. S. Org. Lett. 2019, 21, 8795.
doi: 10.1021/acs.orglett.9b03414 |
| [75] |
Yue, Y.; Shao, H.; Wang, Z.; Wang, K.; Wang, L.; Zhuo, K.; Liu, J. J. Org. Chem. 2020, 85, 11265.
doi: 10.1021/acs.joc.0c01363 |
| [76] |
Liu, J.; Zhang, Y.; Yue, Y.; Wang, Z.; Shao, H.; Zhuo, K.; Lv, Q.; Zhang, Z. J. Org. Chem. 2019, 84, 12946.
doi: 10.1021/acs.joc.9b01586 |
| [77] |
Li, B.; Ni, P.; Huang, H.; Xiao, F.; Deng, G. J. Adv. Synth. Catal. 2017, 359, 4300.
doi: 10.1002/adsc.201701106 |
| [78] |
Ni, P.; Li, B.; Huang, H.; Xiao, F.; Deng, G. J. Green Chem. 2017, 19, 5553.
doi: 10.1039/C7GC02818K |
| [79] |
Liao, Y.; Peng, Y.; Qi, H.; Deng, G. J.; Gong, H.; Li, C. Chem. Commun. 2015, 51, 1031.
doi: 10.1039/C4CC08370A |
| [80] |
Lu, C.; Huang, H.; Tuo, X.; Jiang, P.; Zhang, F.; Deng, G. J. Org. Chem. Front. 2019, 6, 2738.
doi: 10.1039/C9QO00603F |
| [81] |
Chitrala, T.; Rahman, N. K. F. Org Lett, 2020, 22, 1726.
doi: 10.1021/acs.orglett.9b04598 pmid: 32057247 |
| [82] |
Xu, Z.; Deng, G. J.; Zhang, F.; Chen, H.; Huang, H. Org. Lett. 2019, 21, 8630.
doi: 10.1021/acs.orglett.9b03241 |
| [83] |
Chen, J.; Li, G.; Xie, Y.; Liao, Y.; Xiao, F.; Deng, G. J. Org. Lett., 2015, 17, 5870.
doi: 10.1021/acs.orglett.5b03058 |
| [84] |
Jiang, J.; Tuo, X.; Fu, Z.; Huang, H.; Deng, G. J. Org. Biomol. Chem. 2020, 18, 3234.
doi: 10.1039/D0OB00074D |
| [85] |
Nguyen, T. B.; Retailleau, P.; Org. Lett. 2018, 20, 186.
doi: 10.1021/acs.orglett.7b03547 pmid: 29219319 |
| [86] |
Chen, F. J.; Liao, G.; Li, X.; Wu, J. Shi, B. F. Org. Lett. 2014, 16, 5644.
doi: 10.1021/ol5027156 |
| [87] |
Zhang, B.; Liu, D.; Sun, Y.; Zhang, Y.; Feng, J.; Yu, F. Org. Lett. 2021, 23, 8, 3076.
doi: 10.1021/acs.orglett.0c02793 |
| [1] | 李硕, 王明亮, 周来运, 王兰芝. 磁性纳米负载对甲苯磺酸催化串联合成稠合多环的1,5-苯并氧氮杂䓬类化合物[J]. 有机化学, 2023, 43(11): 3977-3988. |
| [2] | 侯学会, 李议慧, 张庆玲, 刘俊桃, 陈亚静. 1,4-吡啶硫内鎓盐在有机合成中的研究与应用[J]. 有机化学, 2023, 43(11): 3844-3860. |
| [3] | 宇世伟, 陈兆华, 陈淇, 林舒婷, 何金萍, 陶冠燊, 汪朝阳. 硫代磺酸酯的合成与应用研究进展[J]. 有机化学, 2022, 42(8): 2322-2330. |
| [4] | 陈幸, 周璇, 姬小趁, 黄华文. 空气氛围下可见光诱导的硫酚氧化脱氢偶联[J]. 有机化学, 2021, 41(12): 4704-4711. |
| [5] | 王柏文, 周永军, 罗时荷, 罗晓燕, 陈伟清, 杨诗敏, 汪朝阳. 非光催化与电化学体系下有机硫试剂参与的烯烃构建C—S键研究进展[J]. 有机化学, 2021, 41(1): 171-184. |
| [6] | 程辉成, 郭鹏虎, 陈冰, 姚嘉伟, 马姣丽, 胡炜杰, 纪红兵. 二苯并噻吩的合成研究进展[J]. 有机化学, 2021, 41(1): 94-104. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||