

过渡金属催化偕二溴烯烃衍生物的偶联反应
收稿日期: 2021-10-19
修回日期: 2021-12-03
网络出版日期: 2021-12-22
基金资助
国家自然科学基金(22078178); 山东省青年创新团队引育计划资助项目.
Transition-Metal Catalyzed Coupling Reactions of gem-Dibromovinyl Derivatives
Received date: 2021-10-19
Revised date: 2021-12-03
Online published: 2021-12-22
Supported by
National Natural Science Foundation of China(22078178); Youth Innovative Talents Attracting and Cultivating Plan of Colleges and Universities in Shandong Province.
梁陆祺 , 张立志 , 彭永利 , 刘会 . 过渡金属催化偕二溴烯烃衍生物的偶联反应[J]. 有机化学, 2022 , 42(4) : 1033 -1060 . DOI: 10.6023/cjoc202110026
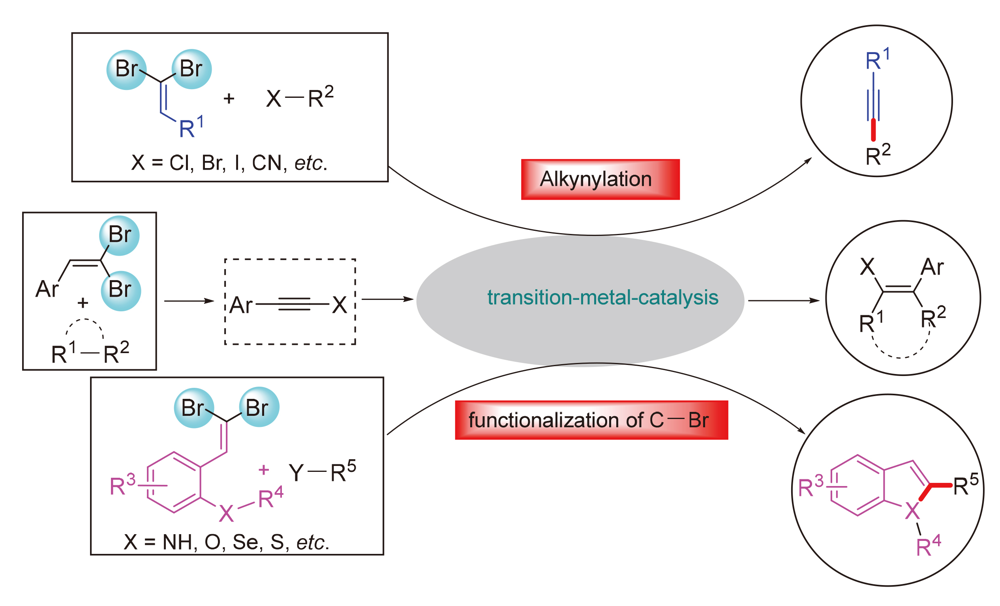
gem-Dibromovinyl derivatives are valuable synthetic tools in organic chemistry, which have been widely applied in the construction of founctionalized molecules. In the past few years, metal-catalyzed (such as Pd, Cu, Ag, Au and Ni) transformations with gem-dibromovinyl derivatives have been developed and become a powerful tool for the synthesis of alkynes, chromenes, indoles, benzindole, benzothiophene and other benzo-fused heterocycles with important physiological activity. The progresses in transition-metal-catalyzed transformations of gem-dibromoviny derivatives since 2013 are summarized.
| [1] | Chelucci, G. Chem. Rev. 2012, 112, 1344 |
| [2] | (a) Legrand, F.; Jouvin, K.; Evano, G. Isr. J. Chem. 2010, 50, 588. |
| [2] | (b) Negishi, E. I. Handbook of Organopalladium Chemistry for Organic Synthesis, Wiley, New York, 2002, 650. |
| [2] | (c) Wang, J. R.; Manabe, K. Synthesis 2009, 1405. |
| [2] | (d) Xu, B.; Ma, S. Chin. J. Org. Chem. 2001, 21, 252. (in Chinese) |
| [2] | ( 许斌, 麻生明, 有机化学, 2001, 21, 252.) |
| [3] | (a) Desal, N. B.; Mckelvie, N.; Ramiraz, F. J. Am. Chem. Soc. 1962, 84, 1745. |
| [3] | (b) Corey, E. J.; Fuchs, P. L. Tetrahedron Lett. 1972, 36, 3769. |
| [4] | Kiruthika, S. E.; Nandakumar, A.; Perumal, P. T. Org. Lett. 2014, 16, 4424. |
| [5] | Xu, W.; Zhang, W.; Zhang, F. Heterocycl. Commun. 2016, 22, 165. |
| [6] | Rao, M. L. N.; Dasgupta, P. RSC Adv. 2015, 5, 65462. |
| [7] | Huang, Z.; Shang, R.; Zhang, Z.-R.; Tan, X.-D.; Xiao, X.; Fu, Y. J. Org. Chem. 2013, 78, 4551. |
| [8] | Meng, T.; Zhang, H.-J.; Xi, Z. Tetrahedron Lett. 2012, 53, 4555. |
| [9] | Aziz, J.; Baladi, T.; Piguel, S. J. Org. Chem. 2016, 81, 4122. |
| [10] | Ji, Y.; Zhong, N.; Kang, Z.; Yan, G.; Zhao, M. Synlett 2018, 29, 209. |
| [11] | Salehi, P.; Shiri, M. Adv. Synth. Catal. 2019, 361, 118. |
| [12] | He, H.-F.; Ye, Y.; Jiang, Y.; Bao, W. J. Chem. Res. 2014, 38, 399. |
| [13] | Shen, C.; Yang, Y.; Liu, Z.; Zhang, Y. Synth. Commun. 2014, 44, 1970. |
| [14] | Kiruthika, S. E.; Perumal, P. T. Org. Lett. 2014, 16, 484. |
| [15] | Zeidan, N.; Bognar, S.; Lee, S.; Lautens, M. Org. Lett. 2017, 19, 5058. |
| [16] | Li, B.; Guo, S.; Zhang, J.; Zhang, X.; Fan, X. J. Org. Chem. 2015, 80, 5444. |
| [17] | Gupta, S.; Koley, D.; Ravikumar, K.; Kundu, B. J. Org. Chem. 2013, 78, 8624. |
| [18] | Song, J.; Chi, X.; Meng, L.; Zhao, P.; Sun, F.; Zhang, D.; Jiao, L.; Liu, Q.; Dong, Y.; Liu, H. Adv. Synth. Catal. 2019, 361, 3599. |
| [19] | Fang, Y.-Q.; Lautens, M. Org. Lett. 2005, 7, 3549. |
| [20] | Zhang, M.; Weng, Z. Org. Lett. 2019, 21, 5838. |
| [21] | Bilheri, F. N.; Pistoia, R. P.; Back, D. F.; Zeni, G. Adv. Synth. Catal. 2017, 359, 4208. |
| [22] | He, H.-F.; Dong, S.; Chen, Y.; Yang, Y.; Le, Y.; Bao, W. Tetrahedron 2012, 68, 3112. |
| [23] | Sayyad, M.; Wani, I. A.; Tiwari, D. P.; Ghorai, M. K. Eur. J. Org. Chem. 2017, 2369. |
| [24] | Zhang, C.; Ban, M.-T.; Zhang, L.-Y.; Zhu, K.; Luo, Z.-Y.; Guo, S.-N.; Cui, D.-M.; Zhang, Y. Org. Lett. 2017, 19, 3947. |
| [25] | Huang, R. Y.; Franke, P. T.; Nicolaus, N.; Lautens, M. Tetrahedron 2013, 69, 4395. |
| [26] | Song, X.; Gao, C.; Zhang, X.; Fan, X. J. Org. Chem. 2018, 83, 15256. |
| [27] | Shi, Y.; Li, M.-S.; Zhang, F.; Chen, B. RSC Adv. 2018, 8, 28668. |
| [28] | Liu, L.; Lv, Y.; Wu, Y.; Gao, X.; Zeng, Z.; Gao, Y.; Tang, G.; Zhao, Y. RSC Adv. 2014, 4, 2322. |
| [29] | Liu, X.; Chen, G.; Zhou, Y.; Liu, P. Tetrahedron Lett. 2018, 59, 3151. |
/
| 〈 |
|
〉 |