

醋酸碘苯促进的脱氢氧化反应合成2-硫芳(烷)基苯酚及10H-吩噻嗪
收稿日期: 2022-01-24
修回日期: 2022-04-12
网络出版日期: 2022-04-29
基金资助
湖北省教育厅科学技术研究(D20192503)
PhI(OAc)2-Promoted Dehydrogenation Oxidation for the Synthesis of 2-(Aryl/alkylthio)phenols and 10H-Phenothiazines
Received date: 2022-01-24
Revised date: 2022-04-12
Online published: 2022-04-29
Supported by
Science and Technology Research Project of Educational Commission of Hubei Province(D20192503)
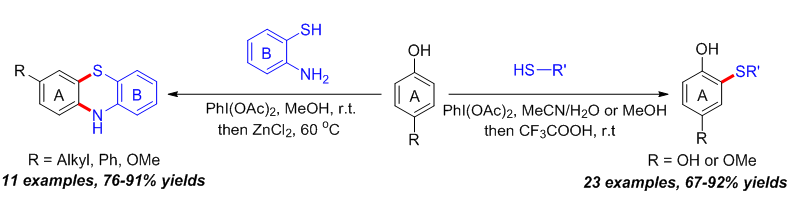
报道了一种醋酸碘苯促进苯酚邻位C(sp2)—H与硫酚S—H之间通过氧化脱氢直接构建C—S键合成2-硫芳(烷)基苯酚衍生物的新方法. 当底物为2-氨基苯硫酚时, 则实现了缩合环化产物10H-吩噻嗪的高效合成. 未见文献报道的新化合物均通过了1H NMR、13C NMR、IR和HRMS的表征, 其中2-((4-溴苯基)硫代)-1,4-苯二酚(3i)的结构还通过了X-ray单晶衍射的证实. 该方法原料易得、条件温和、操作简便, 同时具有良好的原子经济性.
关键词: 2-硫芳(烷)基苯酚; 10H-吩噻嗪; 脱氢氧化; C—S键形成
曹廷舒 , 魏向阳 , 罗敏 , 汪逸飞 , 潘子俊 , 徐程 , 殷国栋 . 醋酸碘苯促进的脱氢氧化反应合成2-硫芳(烷)基苯酚及10H-吩噻嗪[J]. 有机化学, 2022 , 42(7) : 2079 -2088 . DOI: 10.6023/cjoc202201039
A PhI(OAc)2-promoted reaction for the synthesis of 2-(aryl/alkylthio)phenol derivatives has been developed, which was realized via dehydrogenation oxidation between ortho-C(sp2)—H of phenols and S—H of thiophenols for direct formation of C—S bonds. Particularly, this method was also successfully applied for the efficient construction of tricyclic 10H-phenothiazines using 2-aminothiophenols as the substrates. All the newly synthesized products were identified by means of 1H NMR, 13C NMR, IR and HRMS. Besides, 2-((4-bromophenyl)thio)benzene-1,4-diol (3i) was further confirmed by X-ray single crystal diffraction analysis. The method has the advantages of simple and easily available starting materials, mild reaction conditions, simple operation, as well as high atom economy.

| [1] | Smith, G.; Mikkelsen, G.; Eskildsen, J.; Bundgaard, C. Bioorg. Med. Chem. Lett. 2006, 16, 3981. |
| [2] | Amorati, R.; Valgimigli, L.; Panzella, L.; Napolitano, A.; d’Ischia, M. J. Org. Chem. 2013, 78, 9857. |
| [3] | (a) García, A. M.; Brea, J.; Morales-García, J. A.; Perez, D. I.; González, A.; Alonso-Gil, S.; Gracia-Rubio, I.; Ros-Simó, C.; Conde, S.; Cadavid, M. I.; Loza, M. I.; Perez-Castillo, A.; Valverde, O.; Martinez, A.; Gil, C. J. Med. Chem. 2014, 57, 8590. |
| [3] | (b) Dumas, J.; Brittelli, D.; Chen, J.; Dixon, B.; Hatoum-Mokdad, H.; König, G.; Sibley, R.; Witowsky, J.; Wong, S. Bioorg. Med. Chem. Lett. 1999, 9, 2531. |
| [4] | (a) Guzmán-Percástegui, E.; Hernández, D. J.; Castillo, I. Chem. Commun. 2016, 52, 3111. |
| [4] | (b) Fernández-Rodríguez, M. A.; Hartwig, J. F. J. Org. Chem. 2009, 74, 1663. |
| [4] | (c) Lan, M. T.; Wu, W. Y.; Huang, S. H.; Luo, K. L.; Tsai, F. Y. RSC Adv. 2011, 1, 1751. |
| [4] | (d) Xu, X. B.; Liu, J.; Zhang, J. J.; Wang, Y. W.; Peng, Y. Org. Lett. 2013, 15, 550. |
| [4] | (e) Liu, Y.; Huang, B.; Cao, X.; Wu, D.; Wan, J. P. RSC Adv. 2014, 4, 37733. |
| [5] | Liu, X.; Cao, Q.; Xu, W.; Zeng, M. T.; Dong, Z. B. Eur. J. Org. Chem. 2017, 2017, 5795. |
| [6] | Yoshida, S.; Sugimura, Y.; Hazama, Y.; Nishiyama, Y.; Yano, T.; Shimizu, S.; Hosoya, T. Chem. Commun. 2015, 51, 16613. |
| [7] | (a) Xu, R.; Wan, J. P.; Mao, H.; Pan, Y. J. Am. Chem. Soc. 2010, 132, 15531. |
| [7] | (b) Wang, D.; Yu, X.; Wang, L.; Yao, W.; Xu, Z.; Wan, H. Tetrahedron Lett. 2016, 57, 5211. |
| [8] | (a) Wang, D.; Yu, X.; Yao, W.; Hu, W.; Ge, C.; Shi, X. Chem.-Eur. J. 2016, 22, 5543. |
| [8] | (b) Wang, L.; Xie, Y. B.; Huang, N. Y.; Zhang, N. N.; Li, D. J.; Hu, Y. L.; Liu, M. G.; Li, D. S. Adv. Synth. Catal. 2017, 359, 779. |
| [9] | (a) Prasad, C. D.; Balkrishna, S. J.; Kumar, A.; Bhakuni, B. S.; Shrimali, K.; Biswas, S.; Kumar, S. J. Org. Chem. 2013, 78, 1434. |
| [9] | (b) Komeyama, K.; Aihara, K.; Kashihara, T.; Takaki, K. Chem. Lett. 2011, 40, 1254. |
| [10] | (a) Liao, Y.; Jiang, P.; Chen, S.; Qi, H.; Deng, G. J. Green Chem. 2013, 15, 3302. |
| [10] | (b) Ge, W.; Zhu, X.; Wei, Y. Adv. Synth. Catal. 2013, 355, 3014. |
| [10] | (c) Lin, Y. M.; Lu, G. P.; Wang, G. X.; Yi, W. B. Adv. Synth. Catal. 2016, 358, 4100. |
| [11] | (a) Kamimura, A.; Nokubi, T.; Watanabe, R.; Ishikawa, M.; Nasu, K.; Uno, H.; Sumimoto, M. J. Org. Chem. 2014, 79, 1068. |
| [11] | (b) Kamimura, A.; Nokubi, T.; Nasu, K.; Takechi, Y.; Ishihara, Y.; Kato, K.; Noguchi, S.; Watanabe, M.; Shirai, M.; Sumimoto, M.; Uno, H. Chem. Lett. 2012, 41, 950. |
| [11] | (c) Kokorekin, V. A.; Solomatin, Y. A.; Gening, M. L.; Petrosyan, V. A. Mendeleev Commun. 2017, 27, 586. |
| [11] | (d) Farzaliev, V. M.; Allakhverdiev, M. A.; Shamkhalova, S. A.; Rzaeva, I. A. Russ. J. Appl. Chem. 2004, 77, 783. |
| [11] | (e) Carreño, M. C.; García Ruano, J. L.; Urbano, A.; Remor, C. Z.; Arroyo, Y. J. Org. Chem. 2000, 65, 453. |
| [11] | (f) Mbiya, W.; Chipinda, I.; Siegel, P. D.; Mhike, M.; Simoyi, R. H. Chem. Res. Toxicol. 2013, 26, 112. |
| [12] | Rostami, A.; Khakyzadeh, V.; Zolfigol, M. A.; Rostami, A. Mol. Catal. 2018, 452, 260. |
| [13] | Han, D. Y.; Liu, X. P.; Li, R. P.; Xu, D. Z. J. Org. Chem. 2021, 86, 10166. |
| [14] | Liang, G.; Wang, J. H.; Lei, T.; Cheng, Y. Y.; Zhou, C.; Chen, Y. J.; Ye, C.; Chen, B.; Tung, C. H.; Wu, L. Z. Org. Lett. 2021, 23, 8082. |
| [15] | (a) Kamitanaka, T.; Morimoto, K.; Dohi, T.; Kita, Y. Synlett 2019, 30, 1125. |
| [15] | (b) Chandra, G.; Patel, S. ChemistrySelect 2020, 5, 12885. |
| [15] | (c) Taneja, N.; Peddinti, R. K. Tetrahedron Lett. 2016, 57, 3958. |
| [16] | (a) Kamitanaka, T.; Morimoto, K.; Tsuboshima, K.; Koseki, D.; Takamuro, H.; Dohi, T.; Kita, Y. Angew. Chem., Int. Ed. 2016, 55, 15535. |
| [16] | (b) Gao, H.; Xu, Q. L.; Keene, C.; Yousufuddin, M.; Ess, D. H.; Kîrti, L. Angew. Chem., Int. Ed. 2016, 55, 566. |
| [16] | (c) Sharma, S.; Parumala, S. K. R.; Peddinti, R. K. J. Org. Chem. 2017, 82, 9367. |
| [17] | Yin, Z.; Zhang, J.; Wu, J.; Green, R.; Li, S.; Zheng, S. Org. Biomol. Chem. 2014, 12, 2854. |
| [18] | (a) Shen, R.; Zhang, M.; Xiao, J.; Dong, C.; Han, L. B. Green Chem. 2018, 20, 5111. |
| [18] | (b) Zhang, M.; Jia, X.; Zhu, H.; Fang, X.; Ji, C.; Zhao, S.; Han, L. B.; Shen, R. Org. Biomol. Chem. 2019, 17, 2972. |
| [19] | (a) Huang, M.; Huang, D.; Zhu, X.; Wan, Y. Eur. J. Org. Chem. 2015, 2015, 4835. |
| [19] | (b) Lin, Y. M.; Lu, G. P.; Wang, R. K.; Yi, W. B. Org. Lett. 2016, 18, 6424. |
| [19] | (c) Zhang, L.; Wang, H.; Yang, B.; Fan, R. Org. Chem. Front. 2014, 1, 1055. |
| [19] | (d) Gautam, N.; Yadav, A.; Khandelwal, N.; Gautam, D. C. J. Chem. Sci. 2014, 126, 197. |
| [19] | (e) Greiner, I.; Sypaseuth, F. D.; Grun, A.; Karsai, E.; Keglevich, G. Lett. Org. Chem. 2009, 6, 529. |
| [20] | (a) Tan, L.; Liu, W.; Huang, M.; Yu, M. Chin. J. Org. Chem. 2014, 34, 817. (in Chinese) |
| [20] | ( 谭丽泉, 刘卫兵, 黄敏, 余梅, 有机化学, 2014, 34, 817.) |
| [20] | (b) Zhao, G. H.; Li, B. Q.; Wang, S. S.; Liu, M.; Chen, Y.; Wang, B. J. Org. Chem. 2020, 85, 9367. |
| [20] | (c) Jacob, A.; Roy, T.; Kaicharla, T.; Biju, A. T. J. Org. Chem. 2017, 82, 11269. |
| [20] | (d) Liu, L.; Tan, C.; Fan, R.; Wang, Z.; Du, H.; Xu, K.; Tan, J. Org. Biomol. Chem. 2019, 17, 252. |
| [20] | (e) Zhang, H.; Wang, H.; Jiang, Y.; Cao, F.; Gao, W.; Zhu, L.; Yang, Y.; Wang, X.; Wang, Y.; Chen, J.; Feng, Y.; Deng, X.; Lu, Y.; Hu, X.; Li, X.; Zhang, J.; Shi, T.; Wang, Z. Chem.-Eur. J. 2020, 26, 17289. |
| [20] | (f) Yan, Y.; Cui, C.; Li, Z. Chin. J. Org. Chem. 2018, 38, 2501. (in Chinese) |
| [20] | ( 闫溢哲, 崔畅, 李政, 有机化学, 2018, 38, 2501.) |
/
| 〈 |
|
〉 |