

三(邻二甲胺基苄基)钇催化脂肪胺对烯腈的插入串联反应
收稿日期: 2022-03-01
修回日期: 2022-04-05
网络出版日期: 2022-04-29
基金资助
国家自然科学基金(21672005); 国家自然科学基金(20972001)
Reaction of Tandem Addition of Aliphatic Amines to Alkenylnitriles Catalyzed by Tris(o-dimethylaminobenzyl)yttrium
Received date: 2022-03-01
Revised date: 2022-04-05
Online published: 2022-04-29
Supported by
National Natural Science Foundation of China(21672005); National Natural Science Foundation of China(20972001)
侯金松 , 杨高升 . 三(邻二甲胺基苄基)钇催化脂肪胺对烯腈的插入串联反应[J]. 有机化学, 2022 , 42(7) : 2070 -2078 . DOI: 10.6023/cjoc202203002
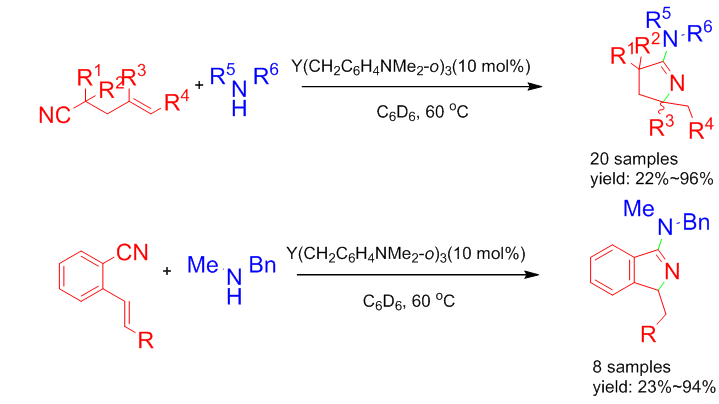
Amidines are nitrogen-containing compounds with very important physiological activities. There are few reports on the synthesis of cyclic amidines catalyzed by rare earth complexes. In this paper, a series of cyclic amidines were synthesized by the tandem reaction of N—H to CN and “C=C” with simple trialkyl rare earth metal complex Y(CH2C6H4NMe2-o)3 as precatalyst with 22%~96% yields. The method has the characteristics of simple catalyst, mild reaction conditions and 100% atomic utilization. It provides a new way for the construction of cyclic amidines.
Key words: rare earth metal complexes; tandem reaction; aliphatic amine; amidine; synthesis
| [1] | (a) Bartsch, A.; Bross, M.; Spiteller, P.; Spiteller, M.; Steglich, W. Angew. Chem., Int. Ed. 2005, 44, 2957. |
| [1] | (b) Inbar, L.; Frolow, F.; Lapidot, A. Eur. J. Biochem. 1993, 214, 897. |
| [1] | (c) Aoyama, T.; Kojima, F.; Imada, C.; Muraoka, Y.; Naganawa, H.; Okami, Y.; Takeuchi, T.; Aoyagi, T. J. Enzyme Inhib. 1995, 8, 223. |
| [1] | (d) Carle, J. S.; Christophersen, C. J. Org. Chem. 1981, 46, 3440. |
| [1] | (e) Green, B. G.; Chabin, R.; Grant, S. K. Biochem. Biophys. Res. Commun. 1996, 225, 621. |
| [2] | (a) Zheng, X. X.; Liu, Y. Y.; Wan, J. P. Chin. J. Org. Chem. 2020, 40, 1891. (in Chinese) |
| [2] | ( 郑茜茜, 刘云云, 万结平, 有机化学, 2020, 40, 1891.) |
| [2] | (b) Banerjee, I.; Sagar, S.; Panda, T. K. Org. Biomol. Chem. 2020, 18, 4231. |
| [2] | (c) Yi, F. P.; Sun, Q. H.; Sun, J.; Fu, C.; Yi, W. Y. J. Org. Chem. 2019, 84, 6780. |
| [2] | (d) Veeranna, K. D.; Das, K. K.; Baskaran, S. Chem. Commun. 2019, 55, 7647. |
| [2] | (e) Dai, Q.; Jiang, Y.; Yu, J. T.; Cheng, J. Chem. Commun. 2015, 51, 16645. |
| [2] | (f) Mishra, D.; Borah, A. J.; Phukan, P.; Hazarika, D.; Phukan, P. Chem. Commun. 2020, 56, 8408. |
| [3] | (a) Zhang, W. H.; Liu, S. F.; Maiga, R. I.; Pelletier, J.; Brown, L. E.; Wang, T. T.; Porco, J. A. J. Am. Chem. Soc. 2019, 141, 1312. |
| [3] | (b) Xu, Y. W.; Zhang, P.; Liu, C. Q.; Lin, C.; Lin, X. Y.; Ke, F. Chin. J. Org. Chem. 2019, 39, 538. (in Chinese) |
| [3] | ( 许贻文, 张鹏, 刘彩琴, 林晨, 林小燕, 柯方, 有机化学, 2019, 39, 538.) |
| [3] | (c) Kretschmer, R. Chem.-Eur. J. 2020, 26, 2099. |
| [4] | (a) Molander, G. A.; Pack, S. K. J. Org. Chem. 2003, 68, 9214. |
| [4] | (b) Wang, J. F.; Xu, F.; Cai, T.; Shen, Q. Org. Lett. 2008, 10, 445. |
| [4] | (c) Spallek, T.; Anwander, R. Dalton Trans. 2016, 45, 16393. |
| [4] | (d) Shi, Y.; Wang, G. Q.; Wang, H.; Deng, B.; Gao, T.; Wang, J.; Guo, H. B.; Wu, M. H.; Sun, S. F. New J. Chem. 2020, 44, 14477. |
| [5] | (a) Zhang, F. J.; Zhang, J.; Zhang, Y.; Hong, J. Q.; Zhou, X. G. Organometallics 2014, 33, 6186. |
| [5] | (b) Shi, X. C.; Nishiura, M.; Hou, Z. M. J. Am. Chem. Soc. 2016, 138, 6147. |
| [5] | (c) Dindar, S.; Kharat, A. N. J. Coord. Chem. 2020, 73, 1954. |
| [6] | (a) Hong, L. C.; Shao, Y. L.; Zhang, L. X.; Zhou, X. G. Chemistry 2014, 20, 8551. |
| [6] | (b) Huang, S. J.; Shao, Y. L.; Zhang, L. X.; Zhou, X. G. Angew. Chem., Int. Ed. 2015, 54, 14452. |
| [6] | (c) Hu, K.; Liu, R. T.; Zhou, X. G. Org. Lett. 2021, 23, 6946. |
| [6] | (d) Ye, P. Q.; Shao, Y. L.; Xie, L. P.; Shen, K. T.; Cheng, T. X.; Chen, J. X. Chem. Asian J. 2018, 13, 3681. |
| [6] | (e) Hou, J. S.; Yang, G. S.; Chai, Z. J. Org. Chem. 2022, 87, 453. |
| [7] | (a) Sheng, E. H.; Wang, S. W.; Yang, G. S.; Zhou, S. L.; Cheng, L.; Zhang, K. H.; Huang, Z. X. Organometallics 2003, 22, 684. |
| [7] | (b) Zhou, S. L.; Wang, S. W.; Yang, G. S.; Liu, X. Y.; Sheng, E. H.; Zhang, K. H.; Cheng, L.; Huang, Z. X. Polyhedron 2003, 22, 1019. |
| [7] | (c) Xie, M. H.; Liu, X. Y.; Wang, S. W.; Liu, L.; Wu, Y. Y.; Yang, G. S.; Zhou, S. L.; Sheng, E. H.; Huang, Z. X. Chin. J. Chem. 2010, 22, 678. |
| [8] | (a) Bradley, D. C.; Ghotra, J. S.; Alan Hart, F. J. Chem. Soc. Dalton Trans. 1973, 1021. |
| [8] | (b) Ling, J.; Peng, H.; Shen, Z. Q. J. Polym. Sci. Part A: Polym. Chem. 2012, 50, 3743. |
| [9] | (a) Arndt, S.; Voth, P.; Spaniol, T. P.; Okuda, J. Organometallics 2000, 19, 4690. |
| [9] | (b) Xie, H. Y.; Wu, C. J.; Cui, D. M.; Wang, Y. J. Organomet. Chem. 2018, 875, 5. |
| [10] | (a) Wayda, A. L.; Atwood, J. L.; Hunter, W. E. Organometallics 1984, 3, 939. |
| [10] | (b) Harder, S. Organometallics 2005, 24, 373. |
| [11] | (a) Manzer, L. E. J. Am. Chem. Soc. 1978, 100, 8068. |
| [11] | (b) Zhang, W. X.; Nishiura, M.; Mashiko, T.; Hou, Z. M. Chem. Eur. J. 2008, 14, 2167. |
| [12] | (a) Martinez, P. H.; Hultzsch, K. C.; Hampel, F. Chem. Commun. 2006, 2221. |
| [12] | (b) Sanjaya, S.; Chiba, S. Tetrahedron 2011, 67, 590. |
| [13] | Xu, H. H.; Kekeli, E. K.; Christian, W. J. Org. Chem. 2008, 73, 7638. |
| [14] | Hahn, B. T.; Tewes, F.; Froehlich, R.; Glorius, F. Angew. Chem., Int. Ed. 2010, 49, 1143. |
/
| 〈 |
|
〉 |