

电化学合成C-磺酰基化合物的研究进展
收稿日期: 2022-05-13
修回日期: 2022-07-14
网络出版日期: 2022-08-25
基金资助
国家自然科学基金(21961042); 广西自然科学基金(2021GXNSFBA075056); 广西高校中青年教师基础能力提升项目(2021KY0587); 广西高校中青年教师基础能力提升项目(2022KY0572); 广西高校中青年教师基础能力提升项目(2021KY0499); 玉林师范学院科研项目(G2021ZK16); 广西农产资源化学与生物技术重点实验室开放课题(2021KF01)
Research Progress of Electrochemical Synthesis of C-Sulfonyl Compounds
Received date: 2022-05-13
Revised date: 2022-07-14
Online published: 2022-08-25
Supported by
National Natural Science Foundation of China(21961042); Natural Science Foundation of Guangxi Province(2021GXNSFBA075056); Basic Ability Improvement Project of Young and Middle-aged Teachers in Guangxi Colleges(2021KY0587); Basic Ability Improvement Project of Young and Middle-aged Teachers in Guangxi Colleges(2022KY0572); Basic Ability Improvement Project of Young and Middle-aged Teachers in Guangxi Colleges(2021KY0499); Research Project of Yulin Normal University(G2021ZK16); Open Project of Guangxi Key Laboratory of Agricultural Resources Chemistry and Biotechnology(2021KF01)
魏琬絜 , 詹磊 , 高雷 , 黄国保 , 马献力 . 电化学合成C-磺酰基化合物的研究进展[J]. 有机化学, 2023 , 43(1) : 17 -35 . DOI: 10.6023/cjoc202205018
Sulfonyl compounds are important organic sulfur compounds, which are widely used in the fields of medicine, pesticides, functional materials and so on. Therefore, efficient strategies for the synthesis of sulfonyl compounds have become the focus of extensive research. Organic electrochemical synthesis is a green, mild and efficient synthesis strategy, which shows great potential in the synthesis of sulfonyl compounds. The reactions of electrochemical synthesis of C-sulfonyl compounds in recent years are introduced. The reactions of electrochemical synthesis of C(sp)-sulfonyl compounds, C(sp2)-sulfonyl compounds and C(sp3)-sulfonyl compounds are classified, summarized and discussed, and the corresponding reaction mechanism is described, so as to provide reference for the application of such reactions in organic synthesis in the future.
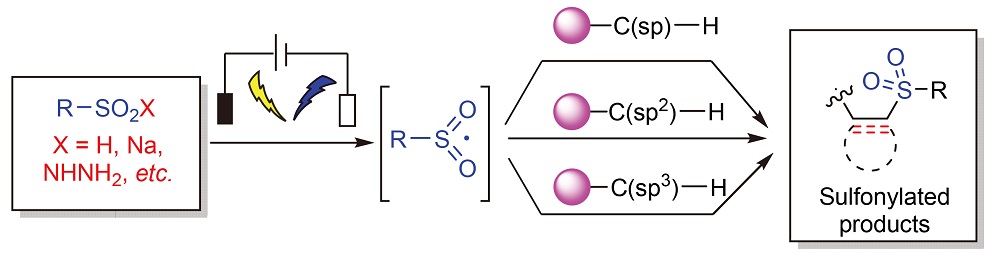
Key words: organic electrochemistry; sulfonyl compounds; sulfonylation
| [1] | (a) Curti, C.; Laget, M.; Carle, A. O.; Gellis, A.; Vanelle, P. Eur. J. Med. Chem. 2007, 42, 880. |
| [1] | (b) Meadows, D. C.; Sanchez, T.; Neamati, N.; North, T. W.; Hague, J. G. Bioorg. Med. Chem. 2007, 15, 1127. |
| [1] | (c) Frankel, B. A.; Bentley, M.; Kruger, R. G.; McCafferty, D. G. J. Am. Chem. Soc. 2004, 126, 3404. |
| [2] | Devendar, P.; Yang, G. F. Top. Curr. Chem. 2017, 375, 82. |
| [3] | (a) Sasabe, H.; Seino, Y.; Kimura, M.; Kido, J. Chem. Mater. 2012, 24, 1404. |
| [3] | (b) Turkoglu, G.; Cinar, M. E.; Ozturk, T. Top. Curr. Chem. 2017, 375, 84. |
| [4] | (a) Zhao, C.; Rakesh, K. P.; Ravidar, L.; Fang, W. Y.; Qin, H. L. Eur. J. Med. Chem. 2019, 162, 679. |
| [4] | (b) Man, H. W.; Schafer, P.; Wong, L. M.; Patterson, R. T.; Corral, L. G.; Raymon, H.; Blease, K.; Leisten, J.; Shirley, M. A.; Tang, Y.; Babusis, D. M.; Chen, R.; Stirling, D.; Muller, G. W. J. Med. Chem. 2009, 52, 1522. |
| [5] | Julia, M.; Paris, J. M. Tetrahedron Lett. 1973, 49, 4833. |
| [6] | (a) Wang, Z.-Q.; Hou, C.; Zhong, Y.-F.; Lu, Y.-X.; Mo, Z.-Y.; Pan, Y.-M.; Tang, H.-T. Org. Lett. 2019, 21, 9841. |
| [6] | (b) He, M.-X.; Mo, Z.-Y.; Wang, Z.-Q.; Cheng, S.-Y.; Xie, R.-R.; Tang, H.-T.; Pan, Y.-M. Org. Lett. 2020, 22, 724. |
| [6] | (c) Meng, X.-J.; Zhong, P.-F.; Wang, Y.-M.; Wang, H.-S.; Tang, H.-T.; Pan, Y.-M. Adv. Synth. Catal. 2020, 362, 506. |
| [6] | (d) Li, Q.-Y.; Cheng, S.-Y.; Tang, H.-T.; Pan, Y.-M. Green Chem. 2019, 21, 5517. |
| [6] | (e) Zhong, P.-F.; Lin, H.-M.; Wang, L.-W.; Mo, Z.-Y.; Meng, X.-J.; Tang, H.-T.; Pan, Y.-M. Green Chem. 2020, 22, 6334. |
| [6] | (f) Wu, Y.; Chen, J.-Y.; Liao, H.-R.; Shu, X.-R.; Duan, L.-L.; Yang, X.-F.; He, W.-M. Green Synth. Catal. 2021, 2, 233. |
| [6] | (g) Li, Q.-Y.; Swaroop, T. R; Hou, C.; Wang, Z.-Q.; Pan, Y.-M.; Tang, H.-T. Adv. Synth. Catal. 2019, 361, 1761. |
| [6] | (h) Chen, N.; Xu, H.-C. Green Synth. Catal. 2021, 2, 165. |
| [6] | (i) He, M.-X.; Zhong, P.-F.; Liu, H.-F.; Ou, C.-H.; Pan, Y.-M.; Tang, H.-T. Green Synth. Catal. 2022, DOI: 10.1016/j.gresc.2022.03.002. |
| [6] | (j) Wang, X.-Y.; Zhong, Y.-F.; Mo, Z.-Y.; Wu, S.-H.; Xu, Y.-L.; Tang, H.-T.; Pan, Y.-M. Adv. Synth. Catal. 2021, 363, 208. |
| [6] | (k) Yang, Z.; Yu, Y.; Lai, L.; Zhou, L.; Ye, K.; Chen, F.-E. Green Synth. Catal. 2021, 2, 19. |
| [6] | (k) Mei, H.; Pajkert, R.; Wang, L.; Li, Z.; R?schenthaler, G.-V.; Han, J. Green Chem. 2020, 22, 3028. |
| [7] | (a) Todoroki, H.; Iwatsu, M.; Urabe, D.; Inoue, M. J. Org. Chem. 2014, 79, 8835. |
| [7] | (b) Kawai, H.; Yuan, Z.; Tokunaga, E.; Shibata, N. Org. Lett. 2012, 14, 5330. |
| [7] | (c) Riddell, N.; Tam, W. J. Org. Chem. 2006, 71, 1934. |
| [7] | (d) Huang, X.; Duan, D.; Zheng, W. J. Org. Chem. 2003, 68, 1958. |
| [7] | (e) Xie, M.; Wang, J.; Gu, X.; Sun, Y.; Wang, S. Org. Lett. 2006, 8, 431. |
| [7] | (f) Zhang, S.; Ye, X.; Wojtas, L.; Hao, W.; Shi, X. Green Synth. Catal. 2021, 2, 82. |
| [8] | (a) Guo, A.; Han, Ji. B.; Zhu, L.; Wei, Y.; Tang, X. Y. Org. Lett. 2019, 21, 2927. |
| [8] | (b) Jin, W.; Wu, M.; Xiong, Z.; Zhu, G. Chem. Commun. 2018, 54, 7924. |
| [8] | (c) Schwarz, J.; K?nig, B. ChemPhotoChem 2017, 1, 237. |
| [8] | (d) Wang, X.; Wu, S.; Zhong, Y.; Wang, Y.; Pan, Y.; Tang, H. Chin. Chem. Lett. 2022, DOI: 10.1016/j.cclet.2022.05.051. |
| [9] | Chen, H.; Zhang, L. Angew. Chem., Int. Ed. 2015, 54, 11775. |
| [10] | Mo, Z.-Y.; Zhang, Y.-Z.; Huang, G.-B.; Wang, X.-Y.; Pan, Y.-M.; Tang, H.-T. Adv. Synth. Catal. 2020, 362, 2160. |
| [11] | Meng, X.; Xu, H.; Cao, X.; Cai, X. M.; Luo, J.; Wang, F.; Huang, S. Org. Lett. 2020, 22, 6827. |
| [12] | Zhong, Q.; Zhao, Y.; Sheng, S.; Chen, J. Synth. Commun. 2020, 50, 161. |
| [13] | Terent’ev, A. O.; Mulina, O. M.; Pirgach, D. A.; Ilovaisky, A. I.; Syroeshkin, M. A.; Kapustina, N. I.; Nikishin, G. I. Tetrahedron 2017, 73, 6871. |
| [14] | Kim, H. S.; Lee, S. Eur. J. Org. Chem. 2019, 41, 6951. |
| [15] | Kong, X.; Yu, K.; Chen, Q.; Xu, B. Asian J. Org. Chem. 2020, 9, 1760. |
| [16] | Zhang, X.; Lu, D.; Wang, Z. Eur. J. Org. Chem. 2021, 30, 4284. |
| [17] | Yuan, Y.; Yu, Y.; Qiao, J.; Liu, P.; Yu, B.; Zhang, W.; Liu, H.; He, M.; Huang, Z.; Lei, A. Chem. Commun. 2018, 54, 11471. |
| [18] | Zhang, Y.-Z.; Mo, Z.-Y.; Wang, H.-S.; Wen, X.-A.; Tang, H.-T.; Pan, Y.-M. Green Chem. 2019, 21, 3807. |
| [19] | Mahanty, k.; Maiti, D.; Sarkar, S. D. J. Org. Chem. 2020, 85, 369. |
| [20] | Wen, J.; Shi, W.; Zhang, F.; Liu, D.; Tang, S.; Wang, H.; Lin, X.-M.; Lei, A. Org. Lett. 2017, 19, 3131. |
| [21] | Lu, F.; Li, J.; Wang, T.; Li, Z.; Jiang, M.; Hu, X.; Pei, H.; Yuan, F.; Lu, L.; Lei, A. Asian J. Org. Chem. 2019, 8, 1838. |
| [22] | Jiang, M.; Yuan, Y.; Wang, T.; Xiong, Y.; Li, J.; Guo, H.; Lei, A. Chem. Commun. 2019, 55, 13852. |
| [23] | Xiao, H.-L.; Yang, C.-W.; Zhang, N.-T.; Zeng, C.-C.; Hu, L.-M.; Tian, H.-Y.; Daniel Little, R. Tetrahedron 2013, 69, 658. |
| [24] | Luo, Y.-C.; Pan, X.-J.; Yuan, G.-Q. Tetrahedron 2015, 71, 2119. |
| [25] | Qian, Peng.; Bi, M.; Su, J.; Zha, Z.; Wang, Z. J. Org. Chem. 2016, 81, 4876. |
| [26] | Nikl, J.; Lips, S.; Schollmeyer, D.; Franke, R.; Waldvogel, S. R. Chem.-Eur. J. 2019, 25, 6891. |
| [27] | Sun, X.; Zhang, F.; Yan, K.; Feng, W.; Sun, X.; Yang, J.; Wen, J. Adv. Synth. Catal. 2021, 363, 3485. |
| [28] | Salmas, R. E.; Seeman, P.; Aksoydan, B.; Erol, I.; Kantarcioglu, I.; Stein, M.; Yurtsever, M.; Durdagi, S. ACS Chem. Neurosci. 2017, 8, 1404. |
| [29] | Nugent, T. C.; El-Shazly, M. Adv. Synth. Catal. 2010, 352, 753. |
| [30] | Gu, Q.; Wang, X.; Liu, X.; Wu, G.; Xie, Y.; Shao, Y.; Zhao, Y.; Zeng, X. Org. Biomol. Chem. 2021, 19, 8295. |
| [31] | Yuan, Y.; Cao, Y.; Lin, Y.; Li, Y.; Huang, Z.; Lei, A. ACS Catal. 2018, 8, 10871. |
| [32] | He, T. J.; Zhong, W. Q.; Huang, J. M. Chem. Commun. 2020, 56, 2735. |
| [33] | Wei, W.-J.; Zhong, Y.-J.; Feng, Y.-F.; Gao, L.; Tang, H.-T.; Pan, Y.-M.; Ma, X.-L.; Mo, Z.-Y. Adv. Synth. Catal. 2021, 364, 726. |
| [34] | Pan, X.; Gao, J.; Yuan, G. Tetrahedron 2015, 71, 5525. |
| [35] | Zheng, M.; Yuan, X.; Cui, Y.; Qiu, J.; Li, G.; Guo, K. Org. Lett. 2018, 20, 7784. |
| [36] | Yavari, I.; Shaabanzadeh, S. Org. Lett. 2020, 22, 464. |
| [37] | Yu, M.; Wang, H.; Gao, Y.; Bu, F.; Cong, H.; Lei, A. Cell Rep. Phys. Sci. 2021, 2, 100476. |
| [38] | Luo, X.; Wang, S.; Lei, A. Adv. Synth. Catal. 2022, 364, 1016. |
/
| 〈 |
|
〉 |