

卤素阴离子催化的立体可控炔烃碳硼化反应研究
收稿日期: 2022-05-18
修回日期: 2022-07-20
网络出版日期: 2022-08-25
基金资助
国家自然科学基金(21542011); 四川省科技厅计划(2020YJ0358); 乐山师范学院科研(DGZZ202018); 乐山师范学院科研(TRCWYFZCH2019006); 乐山师范学院科研(2021SSDJS015); 乐山师范学院科研(2021SSDJS016)
E-Stereospecific 1,1-Carboboration of Terminal Arylalkynes with [IB(C6F5)3]–
Received date: 2022-05-18
Revised date: 2022-07-20
Online published: 2022-08-25
Supported by
National Natural Science Foundation of China(21542011); Science and Technology Department of Sichuan Province(2020YJ0358); Scientific Research Fund of Leshan Normal University(DGZZ202018); Scientific Research Fund of Leshan Normal University(TRCWYFZCH2019006); Scientific Research Fund of Leshan Normal University(2021SSDJS015); Scientific Research Fund of Leshan Normal University(2021SSDJS016)
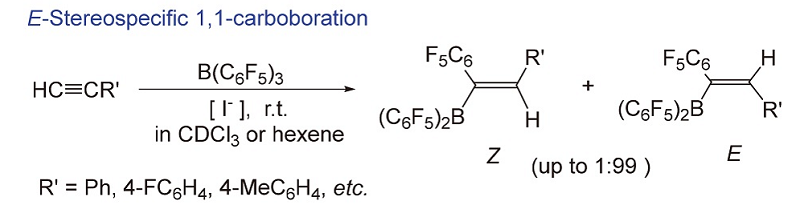
碳硼化反应作为有机合成双官能化反应的策略之一, 是合成各种天然产物、药物分子及精细化工产品重要的方法. 对卤素阴离子催化的立体可控炔烃碳硼化反应进行了详细研究, 通过卤素阴离子种类、用量、反应底物、溶剂、温度和时间等因素对反应活性、立体选择性的影响及其相互作用机制的探索, 发展了一种高立体选择性地制备E式1,1-碳硼化物的新方法; 相关研究表明, 不同的Z、E构型碳硼化物作为催化剂, 对环氧环己烷开环聚合的活性具有明显的差异. 开展相关的立体可控碳硼化反应新方法的研究, 不仅进一步拓展了烯基硼化物在有机双官能团转化反应中的应用, 也对发展新型含硼有机化合物的研究与应用具有重要意义.
田冲 , 孙奇 , 王俊锋 , 陈俏 , 温志国 , Maxim Borzov , 聂万丽 . 卤素阴离子催化的立体可控炔烃碳硼化反应研究[J]. 有机化学, 2023 , 43(1) : 338 -344 . DOI: 10.6023/cjoc202205029
The difunctionalization of alkenes and alkynes is a simple and powerful strategy for the synthesis of various organic compounds and has been used to synthesize various important natural products, drug molecules and fine chemical products. The different effects of halo anions, substrates, solvents, temperature and reaction time to the stereospecific and reactivities of 1,1-carboborations of alkynes and B(C6F5)s were studied, meanwhile the corresponding catalytic mechanisum has been expoled. A convenient large-scale preparation method for the stereoselective (E)-1,1-carboboration products has been developed. The catalytic reactivities of ring-opening polymerization of cyclohexene oxide (CHO) have also been explored with different stereo-carboboranes isolated from 1,1-carboboration reaction, and it is noted that the stereospecificity E- or Z- has shown ambiguously different activities. To develop a stereo-specific approach in the synthesis of vinylboranes will not only be very important for the difunctionalization of alkenes and alkynes, but also for the novel stereospecificity organoborons.

Key words: 1,1-carboboration; stereospecificity; alkynes; borane; reactivity
| [1] | Ding, R.; Liu, Y.-G.; Han, M.-R.; Jiao, W.-Y.; Li, J.-Q.; Tian, Y.-H.; Sun, B.-G. J. Org. Chem. 2018, 83, 12939. |
| [2] | Reddy, D.; Fronczek, F. R.; Watkins, E. B. Org. Lett. 2016, 18, 5620. |
| [3] | Reddy, M. D.; Watkins, E. B. J. Org. Chem. 2015, 80, 11447. |
| [4] | Roy, S.; Roy, S.; Gribble, G. W. Tetrahedron 2012, 68, 9867. |
| [5] | Luszczki, J. J.; Swiader, M. J.; Swiader, K.; Paruszewski, R.; Turski, W. A.; Czuczwar, S. J. Fund. Clin. Pharmacol. 2008, 22, 69. |
| [6] | Liu, Q.-C.; Liu, L.-L.; Ranjala, R.; Hendrik, L.; Guo, Y.; Ye, T. Chin. J. Chem. 2020, 38, 1280. |
| [7] | Bode, J. W.; Sohn, S. S. J. Am. Chem. Soc. 2007, 129, 13798. |
| [8] | Gunanathan, C.; Ben-David, Y.; Milstein, D. Science 2007, 790. |
| [9] | Malawska, B. Curr. Top. Med. Chem. 2005, 5, 69. |
| [10] | Shaabani, A.; Soleimani, E.; Rezayan, A. H. Tetrahedron Lett. 2007, 48, 6137. |
| [11] | Kobayashi, I.; Muraoka, H.; Hasegawa, M.; Saika, T.; Nishida, M.; Kawamura, M.; Ando, R. J. Antimicrob. Chemother. 2002, 50, 129. |
| [12] | Graul, A.; Castaner, J. Drugs Future 1997, 22, 956. |
| [13] | Merino, O.; Santoyo, B. M.; Montiel, L. E.; Jiménez-Vázquez, H. A.; Zepeda, L. G.; Tamariz, J. Tetrahedron Lett. 2010, 51, 3738. |
| [14] | Najera, C.; Sansano, J. M. Chem. Rev. 2007, 107, 4584. |
| [15] | Tanaka, H.; Kuroda, A.; Marusawa, H.; Kino, T.; Goto, T.; Hashimoto, M.; Taga, T. J. Am. Chem. Soc. 1987, 109, 5031. |
| [16] | Montalban, A. G.; Boman, E.; Chang, C. D.; Ceide, S. C.; Dahl, R.; Dalesandro, D.; Delaet, N. G. J.; Erb, E.; Ernst, J. T.; Gibbs, A.; Kahl, J.; Kessler, L.; Kucharski, J.; Lum, C.; Lundstroem, J.; Miller, S.; Nakanishi, H.; Roberts, E.; Saiah, E.; Sullivan, R.; Urban, J.; Wang, Z.; Larson, C. J. Bioorg. Med. Chem. Lett. 2010, 20, 4819. |
| [17] | Ghosh, T.; Maity, P.; Ranu, B. J. Org. Chem. 2018, 83, 11758. |
| [18] | He, Z.-Y.; Guo, J.-Y.; Tian, S.-K. Adv. Synth. Catal. 2018, 360, 1544. |
| [19] | Dong, H.; Hou, M.-F. Chin. J. Org. Chem. 2017, 37, 267. (in Chinese) |
| [19] | (董浩, 侯梅芳, 有机化学, 2017, 37, 267.) |
| [20] | Akerbladh, L.; Schembri, L. S.; Larhed, M.; Odell, L. R. J. Org. Chem. 2017, 82, 12520. |
| [21] | Mane, R. S.; Bhanage, B. M. J. Org. Chem. 2016, 81, 1223. |
| [22] | Sasaki, M.; Ando, M.; Kawahata, M.; Yamaguchi, K.; Takeda, K. Org. Lett. 2016, 18, 1598. |
| [23] | Fan, W.-Z.; Shi, D.-Y.; Feng, B.-N. Tetrahedron Lett. 2015, 56, 4638. |
| [24] | Giustiniano, M.; Mercalli, V.; Cassese, H.; Maro, S. D.; Galli, U.; Novellino, E.; Tron, G. C. J. Org. Chem. 2014, 79, 6006. |
| [25] | Wang, Y.-Y.; Liu, Y.-Y. Acta Chim. Sinica 2019, 77, 418. (in Chinese) |
| [25] | (王昱赟, 刘云云, 化学学报, 2019, 77, 418.) |
| [26] | Liu, L.; Du, L.; Zhang, D.-N.; Du, Y.-F.; Zhao, K. Org. Lett. 2014, 16, 5772. |
| [27] | Mupparapu, N.; Khan, S.; Battula, S.; Kushwaha, M.; Gupta, A. P.; Ahmed, Q. N.; Vishwakarma, R. A. Org. Lett. 2014, 16, 1152. |
| [28] | Cunico, R. F.; Chen, J.-X. Synth. Commun. 2003, 33, 1963. |
| [29] | Yao, Y.; Li, W.-T.; Chen, J.-X. Chin. J. Org. Chem. 2014, 34, 2124. (in Chinese) |
| [29] | (姚远, 李伟东, 陈建新, 有机化学, 2014, 34, 2124.) |
| [30] | Yao, Y.; Tong, W.-T.; Chen, J.-X. Mendeleev Commun. 2014, 24, 176. |
| [31] | Chen, X.-J.; Chen, J.-X. Mendeleev Commun. 2013, 23, 106. |
| [32] | Cao, P.; Wen, X.-P.; Chen, J.-X. Synlett 2017, 28, 353. |
| [33] | Li, W.-D.; Han, S.-H.; Liu, Y.-H.; Chen, J.-X. Chin. J. Org. Chem. 2017, 37, 2423. (in Chinese) |
| [33] | (李伟东, 韩生华, 刘艳红, 陈建新, 有机化学, 2017, 37, 2423.) |
| [34] | Zhang, P.-P.; Chen, W.-W.; Feng, H.; Chen, J.-X. Chin. J. Org. Chem. 2019, 39, 3560. (in Chinese) |
| [34] | (张鹏鹏, 陈雯雯, 冯花, 陈建新, 有机化学, 2019, 39, 3560.) |
| [35] | Li, W.-D.; Han, Y.-L.; Chen, J.-X. Tetrahedron 2017, 73, 5813. |
| [36] | Zhang, P.-P.; Han, S.-H.; Chen, J.-X. Chin. J. Org. Chem. 2020, 40, 1737. (in Chinese) |
| [36] | (张鹏鹏, 韩生华, 陈建新, 有机化学, 2020, 40, 1737.) |
| [37] | Liu, H.; Guo, Q.-L.; Chen, J.-X. Tetrahedron Lett. 2015, 56, 5747. |
| [38] | Guo, Q.-L.; Wen, X.-P.; Chen, J.-X. Tetrahedron 2016, 72, 8117. |
| [39] | Han, Y.-L.; Tong, W.-T.; Liu, H.; Chen, J.-X. Chin. J. Org. Chem. 2018, 38, 1993. (in Chinese) |
| [39] | (韩宇玲, 仝文婷, 刘慧, 陈建新, 有机化学, 2018, 38, 1993.) |
| [40] | Guo, Q. L.; Zhao, M. G.; Chen, J. X. Tetrahedron 2020, 76, 131476. |
| [41] | Tong, W.-T.; Cao, P.; Liu, Y.-H.; Chen, J.-X. J. Org. Chem. 2017, 82, 11603. |
| [42] | Wen, X.-P.; Chen, W. W.; Chen, J.-X. Appl.Organometal. Chem. 2019, 33, e5147. |
| [43] | Zhang, W.-J.; Cao, P.; Guo, Q.-L.; Chen, J.-X. Curr. Org. Synth. 2017, 14, 1067. |
| [44] | Zhang, W.- J; Han, S.-H.; Chen, J.-X. Synth. Commun. 2017, 47, 704. |
| [45] | Ma, F.; Liu, H.; Chen, J.-X. Tetrahedron Lett. 2016, 57, 5246. |
| [46] | Han, Y. L.; Li, Y. P.; Han, S.-H.; Chen, J.-X. Synthesis 2019, 51, 2977. |
| [47] | Cunico, R. F.; Motta, A. R. Org. Lett. 2005, 7, 771. |
| [48] | Asahara, H.; Sofue, A.; Kuroda, Y.; Nishiwaki, N. J. Org. Chem. 2018, 83, 13691. |
| [49] | Weiner, B.; Szymanski, W.; Janssen, D. B.; Minnaard, A. J.; Feringa, B. L. Chem. Soc. Rev. 2010, 39, 1656. |
| [50] | Cheng, R. P.; Gellman, S. H.; DeGrado, W. F. Chem. Rev. 2001, 101, 3219. |
| [51] | Lelais, G.; Seebach, D. Biopolymers 2004, 76, 206. |
| [52] | Vicario, J. L.; Badia, D.; Carrillo, L. Org. Lett. 2001, 3, 773. |
| [53] | Liu, Y.-h.; Ha, Y.-L.; Chen, J.-X. Mendeleev Commun. 2021, 31, 128. |
| [54] | Fornicola, R. S.; Oblinger, E.; Montgomery, J. J. Org. Chem. 1998, 63, 3528. |
| [55] | Baichurin, R. I.; Baichurina, L. V.; Aboskalova, N. I.; Berestovitskaya, V. M. Russ. J. Gen. Chem. 2013, 83, 1764. |
| [56] | Cativiela, C.; Ordonez, M.; Viveros-Ceballos, J. L. Tetrahedron 2020, 76, 130875. |
| [57] | Qian, X.-Y.; Xiong, P.; Xu, H.-C. Acta Chim. Sinica 2019, 77, 879. (in Chinese) |
| [57] | (钱向阳, 熊鹏, 徐海超, 化学学报, 2019, 77, 879.) |
| [58] | Han, Y.-Q.; Zhou, T. Chin. J. Chem. 2020, 38, 527. |
| [59] | Schollkopf, U.; Beckhaus, H. Angew. Chem., Int. Ed. Engl. 1976, 15, 293. |
| [60] | Cunico, R. F.; Pandey, R. K. J. Org. Chem. 2005, 70, 9048. |
/
| 〈 |
|
〉 |