

过渡金属催化的酮羰基导向C—H键官能化反应进展
收稿日期: 2022-05-20
修回日期: 2022-08-09
网络出版日期: 2022-08-25
基金资助
国家自然科学基金(22025109); 国家自然科学基金(21772202); 北京分子科学国家实验室(BNLMS-CXXM-201901); 王宽诚教育基金会资助项目
Advances in Transition-Metal-Catalyzed Keto Carbonyl-Directed C—H Bond Functionalization Reactions
Received date: 2022-05-20
Revised date: 2022-08-09
Online published: 2022-08-25
Supported by
National Natural Science Foundation of China(22025109); National Natural Science Foundation of China(21772202); Beijing National Laboratory for Molecular Sciences(BNLMS-CXXM-201901); K. C. Wong Education Foundation
陈泗林 , 杨芸辉 , 陈超 , 王从洋 . 过渡金属催化的酮羰基导向C—H键官能化反应进展[J]. 有机化学, 2023 , 43(1) : 1 -16 . DOI: 10.6023/cjoc202205033
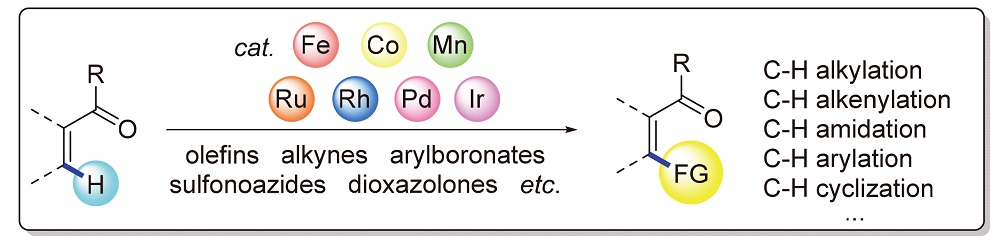
In the past two decades, transition-metal-catalyzed keto carbonyl-directed C—H bond activation has evloved as a powerful and convenient tool for the construction of C—C and C—X (X=N, F, O) bonds at the unconventional reaction sites of ketones. Among them, keto carbonyl-directed C—H bond activation reactions catalyzed by noble metals, involving ruthenium, rhodium, palladium and iridium, have been widely explored, whilst inexpensive 3d metals, such as manganese, iron and cobalt, have gradually emerged as hotspot catalysts in keto carbonyl-directed C—H activation reactions recently. In this review, advances on transition-metal-catalyzed keto carbonyl-directed C—H bond functionalization reactions from 2014 to 2021 are summarized, which are devided by reaction categories such as alkylation, alkenylation, amidation, arylation, cyclization, and so on.
| [1] | (a) Zheng, Q.-Z.; Jiao, N. Tetrahedron Lett. 2014, 55, 1121. |
| [1] | (b) Huang, Z.; Lim, H. N.; Mo, F.; Young, M. C.; Dong, G. Chem. Soc. Rev. 2015, 44, 7764. |
| [1] | (c) Sambiagio, C.; Schonbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M. F.; Wencel- Delord, J.; Besset, T.; Maes, B. U. W.; Schnurch, M. Chem. Soc. Rev. 2018, 47, 6603. |
| [2] | Murai, S.; Kakiuchi, F.; Sekine, S.; Tanaka, Y.; Kamatani, A.; Sonoda, M.; Chatani, N. Nature 1993, 366, 529. |
| [3] | Wang, Z.; Wang, C. Green Synth. Catal. 2021, 2, 66. |
| [4] | Crisenza, G. E.; McCreanor, N. G.; Bower, J. F. J. Am. Chem. Soc. 2014, 136, 10258. |
| [5] | Tsuchikama, K.; Kasagawa, M.; Hashimoto, Y.-K.; Endo, K.; Shibata, T. J. Organomet. Chem. 2008, 693, 3939. |
| [6] | Kimura, N.; Kochi, T.; Kakiuchi, F. J. Am. Chem. Soc. 2017, 139. 14849. |
| [7] | Kimura, N.; Kochi, T.; Kakiuchi, F. Asian J. Org. Chem. 2019, 8, 1115. |
| [8] | Kommagalla, Y.; Srinivas, K.; Ramana, C. V. Chem.-Eur. J. 2014, 20, 7884. |
| [9] | Srinivas, K.; Dangat, Y.; Kommagalla, Y.; Vanka, K.; Ramana, C. V. Chem.-Eur. J. 2017, 23, 7570. |
| [10] | Bettadapur, K. R.; Lanke, V.; Prabhu, K. R. Org. Lett. 2015, 17. 4658. |
| [11] | Sherikar, M. S.; Kapanaiah, R.; Lanke, V.; Prabhu, K. R. Chem. Commun. 2018, 54, 12113. |
| [12] | Han, S. H.; Kim, S.; De, U.; Mishra, N. K.; Park, J.; Sharma, S.; Kwak, J. H.; Han, S.; Kim, H. S.; Kim, I. S. J. Org. Chem. 2016, 81, 12416. |
| [13] | Borah, A. J.; Shi, Z. Chem. Commun. 2017, 53, 3945. |
| [14] | Li, J.-F.; Zhao, R.-F.; Zhou, F.-Q.; She, M.-Y.; Zhang, J.; Yin, B.; Zhang, S.-Y.; Li, J.-L. Org. Chem. Front. 2019, 6, 2607. |
| [15] | Chen, X.; Zheng, G.; Li, Y.; Song, G.; Li, X. Org. Lett. 2017, 19, 6184. |
| [16] | Lee, S. H.; Kundu, A.; Han, S. H.; Mishra, N. K.; Kim, K. S.; Choi, M. H.; Pandey, A. K.; Park, J. S.; Kim, H. S.; Kim, I. S. ACS Omega 2018, 3, 2661. |
| [17] | Shang, R.; Ilies, L.; Nakamura, E. J. Am. Chem. Soc. 2016, 138, 10132. |
| [18] | Santhoshkumar, R.; Mannathan, S.; Cheng, C. H. Org. Lett. 2014, 16, 4208. |
| [19] | Li, G.; Wan, L.; Zhang, G.; Leow, D.; Spangler, J.; Yu, J. Q. J. Am. Chem. Soc. 2015, 137, 4391. |
| [20] | Hu, Y.; Zhou, B.; Chen, H.; Wang, C. Angew. Chem., Int. Ed. 2018, 57, 12071. |
| [21] | Wang, C.; Zhang, Q. Green Synth. Catal. 2022, 3, 287. |
| [22] | Lanke, V.; Bettadapur, K. R.; Prabhu, K. R. Org. Lett. 2016, 18, 5496. |
| [23] | Bakthadoss, M.; Kumar, P. V.; Reddy, T. S. Eur. J. Org. Chem. 2017, 2017, 4439. |
| [24] | Sk, M. R.; Bera, S. S.; Maji, M. S. Adv. Synth. Catal. 2019, 361, 585. |
| [25] | Sk, M. R.; Maji, M. S. Org. Chem. Front. 2020, 7, 19. |
| [26] | Elumalai, K.; Leong, W. K. Tetrahedron Lett. 2018, 59, 113. |
| [27] | (a) Li, C.; Wang, S. M.; Qin, H. L. Org. Lett. 2018, 20, 4699. |
| [27] | (b) Bettadapur, K. R.; Sherikar, M. S.; Lanke, V.; Prabhu, K. R. Asian J. Org. Chem. 2018, 7, 1338. |
| [28] | Dana, S.; Giri, C. K.; Baidya, M. Org. Lett. 2021, 23, 6855. |
| [29] | Hu, F.; Szostak, M. Chem. Commun. 2016, 52, 9715. |
| [30] | Kim, J.; Chang, S. Angew. Chem., Int. Ed. 2014, 53, 2203. |
| [31] | Kong, X.; Xu, B. Org. Lett. 2018, 20, 4495. |
| [32] | Bera, S. S.; Sk, M. R.; Maji, M. S. Chem.-Eur. J. 2019, 25, 1806. |
| [33] | Kim, Y.; Park, J.; Chang, S. Org. Lett. 2016, 18, 1892. |
| [34] | Song, Z.; Antonchick, A. P. Org. Biomol. Chem. 2016, 14, 4804. |
| [35] | Xu, L.; Tan, L.; Ma, D. J. Org. Chem. 2016, 81, 10476. |
| [36] | Hande, A. E.; Prabhu, K. R. J. Org. Chem. 2017, 82, 13405. |
| [37] | Lanke, V.; Prabhu, K. R. Chem. Commun. 2017, 53, 5117. |
| [38] | Chen, S.; Feng, B.; Zheng, X.; Yin, J.; Yang, S.; You, J. Org. Lett. 2017, 19, 2502. |
| [39] | Shi, X.; Xu, W.; Wang, R.; Zeng, X.; Qiu, H.; Wang, M. J. Org. Chem. 2020, 85, 3911. |
| [40] | Wang, F.; Jin, L.; Kong, L.; Li, X. Org. Lett. 2017, 19, 1812. |
| [41] | Shi, P.; Wang, L.; Chen, K.; Wang, J.; Zhu, J. Org. Lett. 2017, 19, 2418. |
| [42] | Yamamoto, T.; Yamakawa, T. RSC Adv. 2015, 5, 105829. |
| [43] | Ogiwara, Y.; Miyake, M.; Kochi, T.; Kakiuchi, F. Organometallics 2017, 36, 159. |
| [44] | Suzuki, I.; Kondo, H.; Kochi, T.; Kakiuchi, F. J. Org. Chem. 2019, 84, 12975. |
| [45] | Zhang, B.; Wang, H. W.; Kang, Y. S.; Zhang, P.; Xu, H. J.; Lu, Y.; Sun, W. Y. Org. Lett. 2017, 19. |
| [46] | Zhang, C.; Rao, Y. Org. Lett. 2015, 17, 4456. |
| [47] | Bruneau, C.; Gramage-Doria, R. Adv. Synth. Catal. 2016, 358, 3847. |
| [48] | Paymode, D. J.; Ramana, C. V. J. Org. Chem. 2015, 80, 11551. |
| [49] | Yang, Y.; Gao, P.; Zhao, Y.; Shi, Z. Angew. Chem., Int. Ed. 2017, 56, 3966. |
| [50] | Rago, A. J.; Dong, G. Green Synth. Catal. 2021, 2, 216. |
| [51] | Shibata, T.; Ryu, N.; Takano, H. Adv. Synth. Catal. 2015, 357, 1131. |
| [52] | Shinde, V. S.; Mane, M. V.; Cavallo, L.; Rueping, M. Chem.-Eur. J. 2020, 26, 8308. |
| [53] | Zhou, S.; Wang, J.; Wang, L.; Song, C.; Chen, K.; Zhu, J. Angew. Chem., Int. Ed. 2016, 55, 9384. |
| [54] | Zhao, Y.; Li, S.; Zheng, X.; Tang, J.; She, Z.; Gao, G.; You, J. Angew. Chem., Int. Ed. 2017, 56, 4286. |
| [55] | Yu, Y.; Wu, Q.; Liu, D.; Hu, L.; Yu, L.; Tan, Z.; Zhu, G. J. Org. Chem. 2019, 84, 7449. |
| [56] | Zhou, B.; Hu, Y.; Liu, T.; Wang, C. Nat. Commun. 2017, 8, 1169. |
| [57] | Liu, T.; Hu, Y.; Yang, Y.; Wang, C. CCS Chem. 2020, 2, 749. |
| [58] | Huo, J.; Yang, Y.; Wang, C. Org. Lett. 2021, 23, 3384. |
| [59] | Dethe, D. H.; C, B. N.; Bhat, A. A. J. Org. Chem. 2020, 85, 7565. |
| [60] | Yanagawa, M.; Harada, S.; Hirose, S.; Nemoto, T. Adv. Synth. Catal. 2021, 363, 2189. |
| [61] | Sk, M. R.; Bera, S. S.; Maji, M. S. Org. Lett. 2018, 20, 134. |
| [62] | Ali, S.; Huo, J.; Wang, C. Org. Lett. 2019, 21, 6961. |
| [63] | Zhang, K.; Khan, R.; Chen, J.; Zhang, X.; Gao, Y.; Zhou, Y.; Li, K.; Tian, Y.; Fan, B. Org. Lett. 2020, 22, 3339. |
| [64] | Lee, P. Y.; Liang, P.; Yu, W. Y. Org. Lett. 2017, 19, 2082. |
| [65] | Zhang, J.; Wu, M.; Fan, J.; Xu, Q.; Xie, M. Chem. Commun. 2019, 55, 8102. |
| [66] | Wu, Q.; Mao, Y. J.; Zhou, K.; Wang, S.; Chen, L.; Xu, Z. Y.; Lou, S. J.; Xu, D. Q. Chem. Commun. 2021, 57, 4544. |
| [67] | Tan, X.; Massignan, L.; Hou, X.; Frey, J.; Oliveira, J. C. A.; Hussain, M. N.; Ackermann, L. Angew. Chem., Int. Ed. 2021, 60, 13264. |
/
| 〈 |
|
〉 |