

二酮的不对称催化还原反应研究进展
收稿日期: 2022-07-30
修回日期: 2022-08-20
网络出版日期: 2022-09-02
基金资助
国家自然科学基金(22071151)
Progress in Asymmetric Catalytic Reduction of Diketones
Received date: 2022-07-30
Revised date: 2022-08-20
Online published: 2022-09-02
Supported by
National Natural Science Foundation of China(22071151)
张宇轩 , 许立民 , 卢岩 , 张兆国 . 二酮的不对称催化还原反应研究进展[J]. 有机化学, 2022 , 42(10) : 3221 -3239 . DOI: 10.6023/cjoc202207045
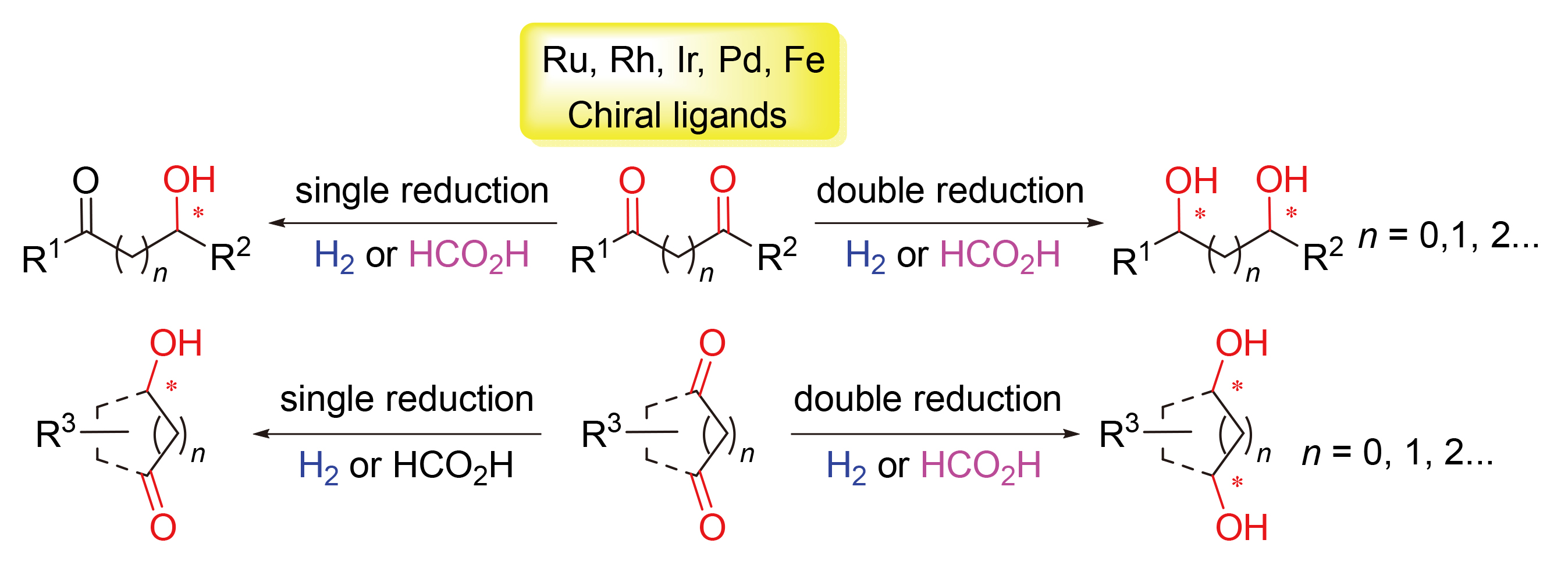
Chiral diols are an integral part of chiral building blocks and synthetic intermediates. Transition metal-catalyzed asymmetric hydrogenation and transfer hydrogenation of diketones are recognized as one of the most straightforward and efficient methods for the preparation of enantiomerically enriched diols. Meanwhile, the mono-reduction product of diketones, chiral hydroxyketones, has wide application in chiral fragment construction as well, among which, desymmetrization of cyclic diketones possesses the capability of establishing multiple chiral centers in one step. In this paper, research progress of asymmetric hydrogenation and transfer hydrogenation of diketone compounds in recent decades is reviewed from the perspective of different substrate types (1,2-/1,3-/1,4-diketones), with emphasis on the influence of coordination mode between substrate and catalyst species on the stereoselectivity. In addition, future challenges and development tendencies in this field are summarized and prospected.
| [1] | Noyori, R.; Ohkuma, T. Angew. Chem., Int. Ed. 2001, 40, 40. |
| [2] | (a) de Vries, J. G.; Elsevier, C. J. Handbook of Homogeneous Hydrogenation, Wiley-VCH, Weinheim, Germany, 2007. |
| [2] | (b) Ma, Y.; Zhang, Y. J.; Zhang, W. Chin. J. Org. Chem. 2007, 27, 289. (in Chinese). |
| [2] | (马元辉, 张勇健, 张万斌, 有机化学, 2007, 27, 289.) |
| [2] | (c) Blaser, H.-U.; Federsel, H.-J. Asymmetric Catalysis on Industrial Scale, 2nd ed., Wiley-VCH, Weinheim, Germany, 2010. |
| [2] | (d) Xie, J.-H.; Zhou, Q.-L. Acta Chim. Sinica 2012, 70, 1427. (in Chinese). |
| [2] | (谢建华, 周其林, 化学学报, 2012, 70, 1427.) |
| [2] | (e) Etayo, P.; Vidal-Ferran, A. Chem. Soc. Rev. 2013, 42, 728. |
| [2] | (f) Wang, Y.; Zhang, Z.; Zhang, W. Chin. J. Org. Chem. 2015, 35, 528. (in Chinese). |
| [2] | (王英杰, 张振锋, 张万斌, 有机化学, 2015, 35, 528.) |
| [2] | (g) Wang, Z.; Zhang, Z.; Liu, Y.; Zhang, W. Chin. J. Org. Chem. 2016, 36, 447. (in Chinese). |
| [2] | (王志惠, 张振锋, 刘燕刚, 张万斌, 有机化学, 2016, 36, 447.) |
| [3] | Noyori, R.; Ohkuma, T.; Kitamura, M.; Takaya, H.; Sayo, N.; Kumobayashi, H.; Akutagawa, S. J. Am. Chem. Soc. 1987, 109, 5856. |
| [4] | (a) Sheldon, R. A. Recl. Trav. Chim. Pays-Bas 1996, 115, 155. |
| [4] | (b) Noyori, R.; Takaya, H. Acc. Chem. Res. 1990, 23, 345. |
| [5] | Rossen, K. Angew. Chem., Int. Ed. 2001, 40, 4611. |
| [6] | (a) Noyori, R.; Koizumi, M.; Ishii, D.; Ohkuma, T. Pure Appl. Chem. 2001, 73, 227. |
| [6] | (b) Dub, P. A.; Gordon, J. C. Dalton Trans. 2016, 45, 6756. |
| [6] | (c) Haack, K.-J.; Hashiguchi, S.; Fujii, A.; Ikariya, T.; Noyori, R. Angew. Chem., Int. Ed. Engl. 1997, 36, 285. |
| [7] | (a) Wu, X.; Xiao, J. Chem. Commun. 2007, 2449. |
| [7] | (b) Ohkuma, T. Proc. Jpn. Acad., Ser. B. 2010, 86, 202. |
| [7] | (c) Štefane, B.; Požgan, F. Catal. Rev. 2014, 56, 82. |
| [7] | (d) Yoshimura, M.; Tanaka, S.; Kitamura, M. Tetrahedron Lett. 2014, 55, 3635. |
| [7] | (e) Li, Y.-Y.; Yu, S.-L.; Shen, W.-Y.; Gao, J.-X. Acc. Chem. Res. 2015, 48, 2587. |
| [7] | (f) Xie, J.-H.; Bao, D.-H.; Zhou, Q.-L. Synthesis 2015, 47, 460. |
| [7] | (g) Li, X.; Zhang, P.; Duan, K.; Wang, J. Chin. J. Org. Chem. 2012, 32, 19. (in Chinese). |
| [7] | (李小娜, 张鹏亮, 段凯, 王家喜, 有机化学, 2012, 32, 19.) |
| [8] | Kitamura, M.; Ohkuma, T.; Inoue, S.; Sayo, N.; Kumobayashi, H.; Akutagawa, S.; Ohta, T.; Takaya, H.; Noyori, R. J. Am. Chem. Soc. 1988, 110, 629. |
| [9] | Fan, Q.; Yeung, C.; Chan, A. S. C. Tetrahedron: Asymmetry 1997, 8, 4041. |
| [10] | Casey, C. P.; Guan, H. J. Am. Chem. Soc. 2007, 129, 5816. |
| [11] | Huang, X.; Li, N.; Geng, Z.; Pan, F.; Wang, X. Chin. J. Chem. 2012, 30, 2657. |
| [12] | Swamy, P. C. A.; Varenikov, A.; de Ruiter, G. Chem. Eur. J. 2020, 26, 2333. |
| [13] | Murata, K.; Okano, K.; Miyagi, M.; Iwane, H.; Noyori, R.; Ikariya, T. Org. Lett. 1999, 1, 1119. |
| [14] | Koike, T.; Murata, K.; Ikariya, T. Org. Lett. 2000, 2, 3833. |
| [15] | Wu, X.; Li, X.; Zanotti-Gerosa, A.; Pettman, A.; Liu, J.; Mills, A. J.; Xiao, J. Chem. Eur. J. 2008, 14, 2209. |
| [16] | Touge, T.; Hakamata, T.; Nara, H.; Kobayashi, T.; Sayo, N.; Saito, T.; Kayaki, Y.; Ikariya, T. J. Am. Chem. Soc. 2011, 133, 14960. |
| [17] | Gladiali, S.; Alberico, E. Chem. Soc. Rev. 2006, 35, 226. |
| [18] | Zhang, H.; Feng, D.; Sheng, H.; Ma, X.; Wan, J.; Tang, Q. RSC Adv. 2014, 4, 6417. |
| [19] | Kišić, A.; Stephan, M.; Mohar, B. Adv. Synth. Catal. 2014, 356, 3193. |
| [20] | Kišić, A.; Stephan, M.; Mohar, B. Adv. Synth. Catal. 2015, 357, 2540. |
| [21] | De Luca, L.; Mezzetti, A. Angew. Chem., Int. Ed. 2017, 56, 11949. |
| [22] | Luca, L. D.; Mezzetti, A. J. Org. Chem. 2020, 85, 5807. |
| [23] | Murayama, H.; Heike, Y.; Higashida, K.; Shimizu, Y.; Yodsin, N.; Wongnongwa, Y.; Jungsuttiwong, S.; Mori, S.; Sawamura, M. Adv. Synth. Catal. 2020, 362, 4655. |
| [24] | Kawano, H.; Ishii, Y.; Saburi, M.; Uchida, Y. J. Chem. Soc., Chem. Commun. 1988, 87. |
| [25] | Shao, L.; Seki, T.; Kawano, H.; Saburi, M. Tetrahedron Lett. 1991, 32, 7699. |
| [26] | Kiegiel, J.; Jóźwik, J.; Woźniak, K.; Jurczak, J. Tetrahedron Lett. 2000, 41, 4959. |
| [27] | Hu, A.; Lin, W. Org. Lett. 2005, 7, 455. |
| [28] | (a) Rychnovsky, S. D.; Griesgraber, G.; Zeller, S.; Skalitzky, D. J. J. Org. Chem. 1991, 56, 5161. |
| [28] | (b) Rychnovsky, S. D. Org. Synth. 2000, 77, 1. |
| [29] | (a) Zhou, S.-Z.; Anné, S.; Vandewalle, M. Tetrahedron Lett. 1996, 37, 7637. |
| [29] | (b) Jung, M. E.; Kretschik, O. J. Org. Chem. 1998, 63, 2975. |
| [29] | (c) Werness, J. B.; Tang, W. Org. Lett. 2011, 13, 3664. |
| [29] | (d) Walleser, P.; Bruckner, R. Org. Lett. 2013, 15, 1294. |
| [29] | (e) Singh, G.; Meyer, A.; Aube, J. J. Org. Chem. 2014, 79, 452. |
| [30] | (a) Bianchini, C.; Barbaro, P.; Scapacci, G.; Zanobini, F. Organometallics 2000, 19, 2450. |
| [30] | (b) Dubrovina, N. V.; Tararov, V. I.; Monsees, A.; Kadyrov, R.; Fischer, C.; Borner, A. Tetrahedron: Asymmetry 2003, 14, 2739. |
| [30] | (c) Brunner, H.; Terfort, A. Tetrahedron: Asymmetry 1995, 6, 919. |
| [31] | (a) Poss, C. S.; Rychnovsky, S. D.; Schreiber, S. L. J. Am. Chem. Soc. 1993, 115, 3360. |
| [31] | (b) Wovkulich, P. M.; Shankaran, K.; Kiegiel, J.; Uskokovic, M. R. J. Org. Chem. 1993, 58, 832. |
| [31] | (c) Schulz, S. Chem. Commun. 1999, 1239. |
| [31] | (d) Juszkiewicz, G.; Jurczak, J. Org. Prep. Proced. Int. 2002, 34, 187. |
| [31] | (e) Arsene, C.; Schulz, S. Org. Lett. 2002, 4, 2869. |
| [31] | (f) Stritzke, K.; Schulz, S.; Nishida, R. Eur. J. Org. Chem. 2002, 2002, 3884. |
| [31] | (g) Wender, P. A.; Mayweg, A. V.; VanDeusen, C. L. Org. Lett. 2003, 5, 277. |
| [31] | (h) Polkowska, J.; Lukaszewicz, E.; Kiegiel, J.; Jurczak, J. Tetrahedron Lett. 2004, 45, 3873. |
| [32] | (a) Balog, A.; Harris, C.; Savin, K.; Zhang, X.-G.; Chou, T.-C.; Danishefsky, S. J. Angew. Chem., Int. Ed. 1998, 37, 2675. |
| [32] | (b) Harris, C. R.; Kuduk, S. D.; Balog, A.; Savin, K.; Glunz, P. W.; Danishefsky, S. J. J. Am. Chem. Soc. 1999, 121, 7050. |
| [32] | (c) Glunz, P. W.; He, L.; Horwitz, S. B.; Chakravarty, S.; Ojima, I.; Chou, T.-C.; Danishefsky, S. J. Tetrahedron Lett. 1999, 40, 6895. |
| [32] | (d) Stachel, S. J.; Chappell, M. D.; Lee, C. B.; Danishefsky, S. J.; Chou, T.-C.; He, L.; Horwitz, S. B. Org. Lett. 2000, 2, 1637. |
| [32] | (e) Lee, C. B.; Chou, T.-C.; Zhang, X.-G.; Wang, Z.-G.; Kuduk, S. D.; Chappell, M. D.; Stachel, S. J.; Danishefsky, S. J. J. Org. Chem. 2000, 65, 6525. |
| [32] | (f) Haydl, A. M.; Breit, B. Chem. Eur. J. 2017, 23, 541. |
| [33] | Mezzetti, A.; Consiglio, G. J. Chem. Soc., Chem. Commun. 1991, 1675. |
| [34] | Mezzetti, A.; Tschumper, A.; Consiglio, G. J. Chem. Soc., Dalton Trans. 1995, 49. |
| [35] | Pini, D.; Mandoli, A.; Iuliano, A.; Salvadori, P. Tetrahedron: Asymmetry 1995, 6, 1031. |
| [36] | Blanc, D.; Ratovelomanana-Vidal, V.; Marinetti, A.; Genêt, J.-P. Synlett 1999, 1999, 480. |
| [37] | Michaud, G.; Bulliard, M.; Ricard, L.; Genêt, J. P.; Marinetti, A. Chem. Eur. J. 2002, 8, 3327. |
| [38] | (a) Duprat de Paule, S.; Jeulin, S.; Ratovelomanana-Vidal, V.; Genêt, J.-P.; Champion, N.; Deschaux, G.; Dellis, P. Org. Process Res. Dev. 2003, 7, 399. |
| [38] | (b) Duprat de Paule, S.; Jeulin, S.; Ratovelomanana-Vidal, V.; Genêt, J.-P.; Champion, N.; Dellis, P. Eur. J. Org. Chem. 2003, 1931. |
| [39] | Genêt, J.-P.; Ratovelomanana-Vidal, V.; Jeulin, S.; Champion, N.; Dellis, P. Synthesis 2005, 3666. |
| [40] | Clarke, M. L.; France, M. B.; Knight, F. R.; Frew, J. J. R.; Roff, G. J. Synlett 2007, 1739. |
| [41] | Jeulin, S.; Duprat de Paule, S.; Ratovelomanana-Vidal, V.; Genêt, J. P.; Champion, N.; Dellis, P. Angew. Chem., Int. Ed. 2004, 43, 320. |
| [42] | Kesselgruber, M.; Lotz, M.; Martin, P.; Melone, G.; Muller, M.; Pugin, B.; Naud, F.; Spindler, F.; Thommen, M.; Zbinden, P.; et al. Chem Asian J 2008, 3, 1384. |
| [43] | Li, W.; Fan, W.; Ma, X.; Tao, X.; Li, X.; Xie, X.; Zhang, Z. Chem. Commun. 2012, 48, 8976. |
| [44] | Li, W.; Lu, B.; Xie, X.; Zhang, Z. Org. Lett. 2019, 21, 5509. |
| [45] | Yamamura, T.; Nakatsuka, H.; Tanaka, S.; Kitamura, M. Angew. Chem., Int. Ed. 2013, 52, 9313. |
| [46] | Yu, C.-B.; Wang, H.-D.; Song, B.; Shen, H.-Q.; Fan, H.-J.; Zhou, Y.-G. Sci. China Chem. 2020, 63, 215. |
| [47] | (a) Ireland, T.; Grossheimann, G.; Wieser-Jeunesse, C.; Knochel, P. Angew. Chem., Int. Ed. 1999, 38, 3212. |
| [47] | (b) Ireland, T.; Tappe, K.; Grossheimann, G.; Knochel, P. Chem. Eur. J. 2002, 8, 843. |
| [47] | (c) Lotz, M.; Polborn, K.; Knochel, P. Angew. Chem., Int. Ed. 2002, 41, 4708. |
| [47] | (d) Tappe, K.; Knochel, P. Tetrahedron: Asymmetry 2004, 15, 91. |
| [48] | Spindler, F.; Malan, C.; Lotz, M.; Kesselgruber, M.; Pittelkow, U.; Rivas-Nass, A.; Briel, O.; Blaser, H. U. Tetrahedron: Asymmetry 2004, 15, 2299. |
| [49] | (a) Sturm, T.; Weissensteiner, W.; Spindler, F. Adv. Synth. Catal. 2003, 345, 160. |
| [49] | (b) Wang, Y.; Sturm, T.; Steurer, M.; Arion, V. B.; Mereiter, K.; Spindler, F.; Weissensteiner, W. Organometallics 2008, 27, 1119. |
| [50] | (a) Zirakzadeh, A.; Gross, M. A.; Wang, Y.; Mereiter, K.; Spindler, F.; Weissensteiner, W. Organometallics 2013, 32, 1075. |
| [50] | (b) Zirakzadeh, A.; Gross, M. A.; Wang, Y.; Mereiter, K.; Weissensteiner, W. Organometallics 2014, 33, 1945. |
| [51] | (a) Fukuzawa, S.; Oki, H.; Hosaka, M.; Sugasawa, J.; Kikuchi, S. Org. Lett. 2007, 9, 5557. |
| [51] | (b) Oki, H.; Oura, I.; Nakamura, T.; Ogata, K.; Fukuzawa, S. Tetrahedron: Asymmetry 2009, 20, 2185. |
| [52] | Espino, G.; Xiao, L.; Puchberger, M.; Mereiter, K.; Spindler, F.; Manzano, B. R.; Jalon, F. A.; Weissensteiner, W. Dalton Trans. 2009, 2751. |
| [53] | Gong, Q.; Wen, J.; Zhang, X. Chem. Sci. 2019, 10, 6350. |
| [54] | Morken, J. P.; Russell, A. E; Taylor, S. J. In Encyclopedia of Reagents for Organic Synthesis, Eds.: John, W.; Sons, L., John Wiley & Sons, Ltd., New York, 2018, p. 1. |
| [55] | Blandin, V.; Carpentier, J.-F.; Mortreux, A. New J. Chem. 2000, 24, 309. |
| [56] | Marinetti, A.; Jus, S.; Genêt, J. P.; Ricard, L. J. Organomet. Chem. 2001, 624, 162. |
| [57] | Imamoto, T.; Nishimura, M.; Koide, A.; Yoshida, K. J. Org. Chem. 2007, 72, 7413. |
| [58] | (a) Xie, J. H.; Liu, X. Y.; Yang, X. H.; Xie, J. B.; Wang, L. X.; Zhou, Q. L. Angew. Chem., Int. Ed. 2012, 51, 201. |
| [58] | (b) Yang, X. H.; Xie, J. H.; Zhou, Q. L. Org. Chem. Front. 2014, 1, 190. |
| [59] | Chen, Y.-C.; Deng, J.-G.; Wu, T.-F.; Cui, X.; Jiang, Y.-Z.; Choi, M. C. K.; Chan, A. S. C. Chin. J. Chem. 2001, 19, 807. |
| [60] | Cossy, J.; Eustache, F.; Dalko, P. I. Tetrahedron Lett. 2001, 42, 5005. |
| [61] | Eustache, F.; Dalko, P. I.; Cossy, J. Org. Lett. 2002, 4, 1263. |
| [62] | Eustache, F.; Dalko, P. I.; Cossy, J. J. Org. Chem. 2003, 68, 9994. |
| [63] | Watanabe, M.; Murata, K.; Ikariya, T. J. Org. Chem. 2002, 67, 1712. |
| [64] | Chen, Y. C.; Wu, T. F.; Deng, J. G.; Liu, H.; Cui, X.; Zhu, J.; Jiang, Y. Z.; Choi, M. C.; Chan, A. S. J. Org. Chem. 2002, 67, 5301. |
| [65] | Soni, R.; Collinson, J. M.; Clarkson, G. C.; Wills, M. Org. Lett. 2011, 13, 4304. |
| [66] | Kuang, L.; Liu, L. L.; Chiu, P. Chem. Eur. J. 2015, 21, 14287. |
| [67] | Cotman, A. E.; Cahard, D.; Mohar, B. Angew. Chem., Int. Ed. 2016, 55, 5294. |
| [68] | Yu, C.-B.; Song, B.; Chen, M.-W.; Shen, H.-Q.; Zhou, Y.-G. Org. Lett. 2019, 21, 9401. |
| [69] | Ding, Y.-X.; Zhu, Z.-H.; Wang, H.; Yu, C.-B.; Zhou, Y.-G. Sci. China Chem. 2021, 64, 232. |
| [70] | Wang, J.; Shao, P.-L.; Lin, X.; Ma, B.; Wen, J.; Zhang, X. Angew. Chem., Int. Ed. 2020, 59, 18166. |
| [71] | Clay, D. R.; Rosenberg, A. G.; McIntosh, M. C. Tetrahedron: Asymmetry 2011, 22, 713. |
| [72] | Fang, Z.; Wills, M. J. Org. Chem. 2013, 78, 8594. |
| [73] | Echeverria, P.-G.; Férard, C.; Phansavath, P.; Ratovelomanana-Vidal, V. Catal. Commun. 2015, 62, 95. |
| [74] | Zheng, L.-S.; Llopis, Q.; Echeverria, P.-G.; Férard, C.; Guillamot, G.; Phansavath, P.; Ratovelomanana-Vidal, V. J. Org. Chem. 2017, 82, 5607. |
| [75] | Hong, Y.; Chen, J.; Zhang, Z.; Liu, Y.; Zhang, W. Org. Lett. 2016, 18, 2640. |
| [76] | Zhang, F.-H.; Zhang, F.-J.; Li, M.-L.; Xie, J.-H.; Zhou, Q.-L. Nat. Catal. 2020, 3, 621. |
/
| 〈 |
|
〉 |