

芳炔参与的三组分芳基化反应进展
收稿日期: 2022-06-06
修回日期: 2022-08-30
网络出版日期: 2022-10-27
基金资助
国家自然科学基金(22061038); 国家自然科学基金(22067018); 甘肃省自然科学基金(20YF3GA023); 甘肃省自然科学基金(20JR5RA210)
Recent Progress on Arylation with Aryne through Three-Component Reaction
Received date: 2022-06-06
Revised date: 2022-08-30
Online published: 2022-10-27
Supported by
National Natural Science Foundation of China(22061038); National Natural Science Foundation of China(22067018); Natural Science Foundation of Gansu Province(20YF3GA023); Natural Science Foundation of Gansu Province(20JR5RA210)
陈东平 , 杨春红 , 李明 , 赵国孝 , 王文鹏 , 王喜存 , 权正军 . 芳炔参与的三组分芳基化反应进展[J]. 有机化学, 2023 , 43(2) : 503 -525 . DOI: 10.6023/cjoc202206006
The three component reaction has the advantages of simple operation and efficient reaction, in line with the principles of atomic economy and green environmental protection. At present, the arylation without transition metal catalyst is mainly divided into three types of as following: (1) the direct arylation, (2) the insertion of aryne into σ-bond or π-bond, and (3) the three-component reactions involving aryne. Compared with the direct arylation and the insertion of aryne into σ-bond or π-bond, the mechanism of three component reactions involving aryne is still unclear, and the published reviews are still lacking. In order to facilitate the scientific researchers to consult and understand the three-component reaction involved in aryne, the progresses in the three-component reaction involving aryne in recent years are reviewed.
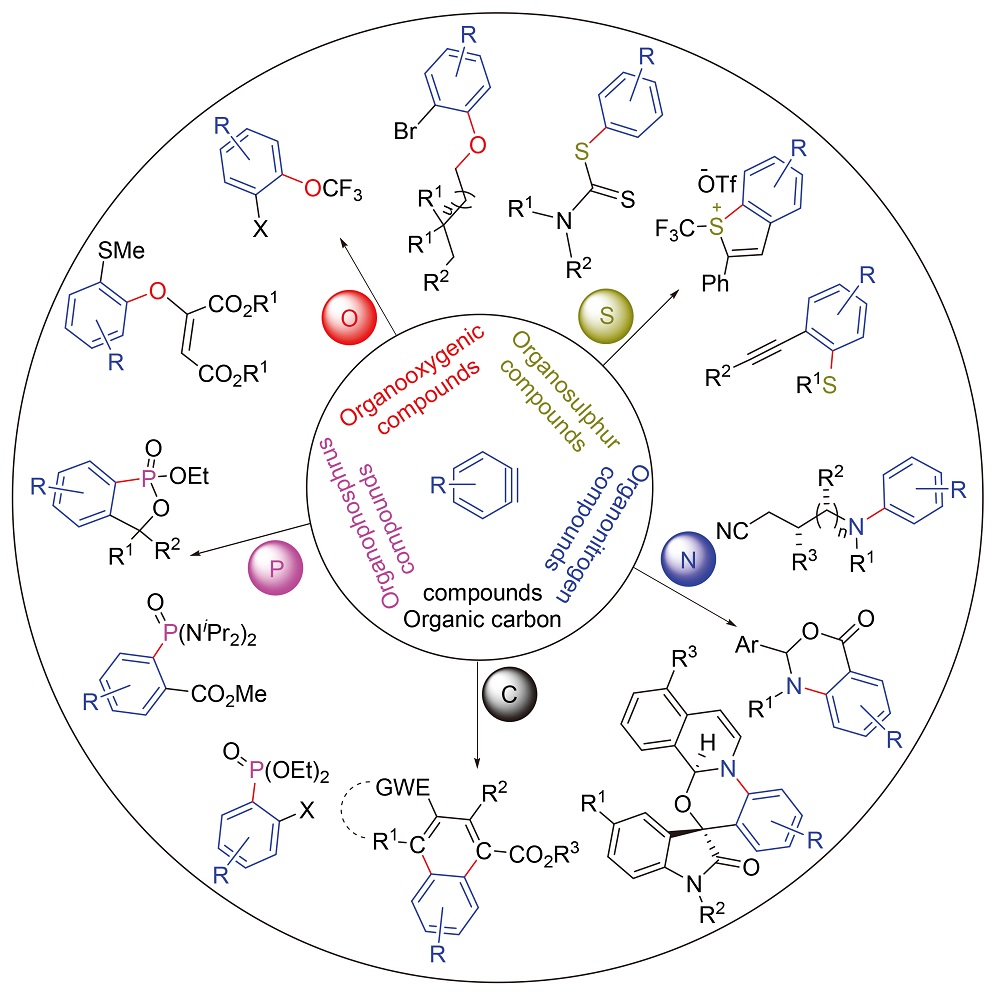
Key words: aryne; three component reaction; carbon and heteroatom; arylation
| [1] | (a) Demmer, C. S.; KrogsgaardLarsen, N.; Bunch, L. Chem. Rev. 2011, 111, 7981. |
| [1] | (b) Montchamp, J. L. Acc. Chem. Res. 2014, 47, 77. |
| [1] | (c) Zhang, J.; Ding, D.; Wei, Y.; Xu, H. Chem. Sci. 2016, 7, 2870. |
| [2] | (a) Otzen, T.; Wempe, E. G.; Kunz, B.; Seydel, J. K. J. Med. Chem. 2004, 47, 240. |
| [2] | (b) Zhang, K.; Xu, X.; Qing, F. Chin. J. Org. Chem. 2015, 35, 556. (in Chinese) |
| [2] | (张柯, 徐修华, 卿凤翎, 有机化学, 2015, 35, 556.) |
| [2] | (c) Lee, C. F.; Liu, Y. C.; Badsara, S. S. Chem.-Asian J. 2014, 9, 706. |
| [2] | (d) Chauhan, P.; Mahajan, S.; Enders, D. Chem. Rev. 2014, 114, 8807. |
| [3] | (a) Tang, W.; Zhang, X. Chem. Rev. 2003, 103, 3029. |
| [3] | (b) Martin, R.; Buchwald, S. L. Acc. Chem. Res. 2008, 41, 1461. |
| [4] | (a) Wang, Y.; Wu. M.; Ding, Y. Chin. J. Org. Chem. 2010, 30, 757. (in Chinese) |
| [4] | (王勇, 吴梅, 丁贻祥, 有机化学, 2010, 30, 757.) |
| [4] | (b) Xu, Q.; Jia, X.; Li, X.; Sun, Q.; Zhou, Y.; Yin, S.; Han, L. Chin. J. Org. Chem. 2014, 34, 1340. (in Chinese) |
| [4] | (徐清, 贾小娟, 李晓慧, 孙清, 周永波, 尹双凤, 韩立彪, 有机化学, 2014, 34, 1340.) |
| [4] | (c) Jablonkai, E.; Keglevich, G. Curr. Org. Synth. 2014, 11, 429. |
| [4] | (d) Jablonkai, E.; Keglevich, G. Org. Prep. Proced. Int. 2014, 46, 281. |
| [5] | (a) Yang, M.; Pei, J.; Yan, G.; Weng, Q. Chin. J. Org. Chem. 2013, 33, 343. (in Chinese) |
| [5] | (杨明华, 裴吉, 严国兵, 翁秋月, 有机化学, 2013, 33, 343.) |
| [5] | (b) Umierski, N.; Manolikakes, G. Org. Lett. 2013, 15, 188. |
| [5] | (c) Johnson, M. W.; Bagley, S. W.; Mankad, N. P.; Mascitti, V.; Toste, F. D. Angew. Chem., Int. Ed. 2014, 53, 4404. |
| [5] | (d) RichardsTaylor, C. S.; Blakemore, D. C.; Willis, M. C. Chem. Sci. 2014, 52, 12679. |
| [6] | (a) Wenk, H. H.; Winkler, M.; Sander, W. Angew. Chem., Int. Ed. 2003, 42, 502. |
| [6] | (b) Okuma, K. Heterocycles 2012, 85, 515. |
| [6] | (c) Bhojgude, S. S.; Biju, A. T. Angew. Chem., Int. Ed. 2012, 51, 1520. |
| [6] | (d) Wu, C.; Shi, F. Asian J. Org. Chem. 2013, 2, 116. |
| [6] | (e) Modha, S. G.; Mehta, V. P.; VanderEycken, E. V. Chem. Soc. Rev. 2013, 42, 5042. |
| [7] | Himeshima, Y.; Sonoda, T.; Kobayashi, H. Chem. Lett. 1983, 12, 1211. |
| [8] | Matsuzawa, T.; Yoshida, S.; Hosoya, T. Tetrahedron Lett. 2018, 59, 4197. |
| [9] | (a) Chen, L.; Zhang, C.; Wen, C.; Zhang, K.; Liu, W.; Chen, Q. Catal. Commun. 2015, 65, 81. |
| [9] | (b) Chen, Q.; Zhang, C.; Du, Z.; Chen, H.; Zhang, K. Tetrahedron Lett. 2015, 56, 2094. |
| [9] | (c) Wen, C.; Chen, Q.; He, Z.; Yan, X.; Zhang, C.; Du, Z.; Zhang, K. Tetrahedron Lett. 2015, 56, 5470. |
| [9] | (d) Chen, Q.; Yan, X.; Du, Z.; Zhang, K.; Wen, C. J. Org. Chem. 2016, 81, 276. |
| [9] | (e) Chen, Q.; Yan, X.; Wen, C.; Zeng, J.; Huang, Y.; Liu, X.; Zhang, K. J. Org. Chem. 2016, 81, 9476. |
| [9] | (f) Huang, Y. T.; Chen, Q. Chin. J. Org. Chem. 2020, 40, 4087. (in Chinese) |
| [9] | (黄远婷, 陈迁, 有机化学, 2020, 40, 4087.) |
| [9] | (g) Shi, J. R.; Li, L. G.; Li, Y. Chem. Rev. 2021, 121, 3892. |
| [9] | (h) Kashmiri, N.; Pranjal, G. Synlett 2020, 31, 750. |
| [10] | (a) Sourav, G.; Daesung, L. Synth. Catal. 2021, 363, 657. |
| [10] | (b) Kashmiri, N.; Pranjal, G. Org. Biomol. Chem. 2020, 18, 9549. |
| [11] | Yoshida, S.; Hosoya, T. Chem. Lett. 2013, 42, 583. |
| [12] | Bhunia, A.; Kaicharla, T.; Porwal, D.; Gonnade, R. G.; Biju, A. T. Chem. Commun. 2014, 50, 11389. |
| [13] | Bhunia, A.; Roy, T.; Gonnade, R. G.; Biju, A. T. Org. Lett. 2014, 16, 5132. |
| [14] | Bhattacharjee, S.; Raju, A.; Gaykar, R. N.; Gonnade, R. G.; Roy, T.; Biju, A. T. J. Org. Chem. 2020, 15, 2203. |
| [15] | Xie, P.; Yang, S.; Guo, Y.; Cai, Z.; Dai, B.; He, L. J. Org. Chem. 2020, 85, 8872. |
| [16] | Huang, Y. T.; Chen, Q. J. Org. Chem. 2021, 86, 7010. |
| [17] | Jin, J. H.; Kim, J.; Han, S. J. Org. Lett. 2022, 24, 2192. |
| [18] | Liu, F. L.; Chen, J. R.; Zou, Y. Q.; Wei, Q.; Xiao, W. J. Org. Lett. 2014, 16, 3768. |
| [19] | Hazarika, H.; Neog, K.; Sharma, A.; Das, B.; Gogoi, P. J. Org. Chem. 2019, 84, 5846. |
| [20] | Lou, M. M.; Wang, H.; Li, Z. Q.; Guo, X. S.; Zhang, F. G.; Wang, B. J. Org. Chem. 2016, 81, 5915. |
| [21] | Qiu, D.; Gu, R.; Wang, J.; Shi, J.; Li, Y. J. Am. Chem. Soc. 2016, 138, 10814. |
| [22] | Dai, L.; Tan, M.; Lan, Y.; Li, Y. J. Am. Chem. Soc. 2021, 143, 10530. |
| [23] | Hazaria, H.; Gogoi, P. Org. Biomol. Chem. 2020, 18, 2727. |
| [24] | Arbuzov, B. A. Pure Appl. Chem. 1964, 9, 307. |
| [25] | Xu, H. D.; Cai, M. Q.; He, W. J.; Hu, W. H.; Shen, M. H. RSC Adv. 2014, 4, 7623. |
| [26] | (a) Lin, W.; Sapountzis, I.; Knochel, P. Angew. Chem., Int. Ed. 2005, 44, 4258. |
| [26] | (b) Yoshida, S.; Hosoya, T. Tetrahedron Lett. 2018, 59, 4197. |
| [26] | (c) Fan, R.; Tan, C.; Liu, Y. G.; Zhao, X. W.; Tian, J. J.; Yoshidab, H. Chin. Chem. Lett. 2021, 32, 299 |
| [27] | Peng, X. L.; Ma, C.; Xu, Z. H. Org. Lett. 2016, 18. 4154. |
| [28] | Zeng, Y.; Hu, J. B. Org. Lett. 2016, 18, 856. |
| [29] | Tadross, P. M.; Stoltz, B. M. Chem. Rev. 2012. 112. 3550. |
| [30] | Zheng, T.; Tan, J.; Fan, R.; Su, S.; Liu, B.; Tan, C.; Xu, K. Chem. Commun. 2018, 54, 1303. |
| [31] | Fan, R.; Liu, B.; Zheng, T.; Xu, K.; Tan, C.; Zeng, T.; Su, S.; Tan, J. Chem. Commun. 2018, 54, 7081. |
| [32] | Jian, H.; Wang, Q.; Wang, W. H.; Li, Z. J.; Gu, C. Z.; Dai, B.; He, L. Tetrahedron 2018, 74, 2876. |
| [33] | Gaykar, R.; Guin, A.; Bhattacharjee, S.; Biju, A. T. Org. Lett. 2019, 21, 9613. |
| [34] | Subrata, B.; Akkattu, T.; Biju, A. T. Org. Lett. 2020, 22, 9097. |
| [35] | Hu, Y. F.; Huang, Y. T.; Zhao, X.; Li, X. W.; Chen, Q. Org. Biomol. Chem. 2021, 19, 7066. |
| [36] | Garima, J.; Biju, A. T. Org. Lett. 2021, 23, 9083. |
| [37] | Meng, D. P.; Ni, C. F; Zhou, M.; Li, Y.; Hu, J. B. Chem.-Eur. J. 2022, 28, e202104395. |
| [38] | Okuma, K.; Hino, H.; Sou, A.; Nagahora, N.; Shioji, K. Chem. Lett. 2009, 38, 1030. |
| [39] | Okuma, K.; Fukuzaki, Y.; Nojima, A.; Shioji, K.; Yokomori, Y. Tetrahedron Lett. 2008, 49, 3063. |
| [40] | Okuma, K.; Fukuzaki, Y.; Shioji, K.; Yokomori, Y. Bull. Chem. Soc. Ethiop. 2010, 83, 1238. |
| [41] | Yoshida, H.; Asatsu, Y.; Mimura, Y.; Ito, Y.; Ohshita, J.; Takaki, K. Angew. Chem., Int. Ed. 2011, 50, 9676. |
| [42] | Thangaraj, M.; Bhojgude, S. S.; Mane, M. V.; Biju, A. T. Chem. Commun. 2016, 52, 1665. |
| [43] | Li, P.; Wu, C.; Zhao, J.; Li, Y.; Xue, W.; Shi, F. Can. J. Chem. 2013, 91, 43. |
| [44] | Zhou, C.; Wang, J.; Jin, J.; Lu, P.; Wang, Y. Eur. J. Org. Chem. 2014, 14, 1832. |
| [45] | Liu, F.; Yang, H.; Hu, X.; Jiang, G. Org. Lett. 2014, 16, 6408. |
| [46] | Sharma, A.; Gogoi, P. ChemistrySelect 2017, 2, 11801. |
| [47] | Wen, L.W.; Man, N. N.; Yuan, W. K.; Li, M. J. Org. Chem. 2016, 81, 5942. |
| [48] | Neog, K.; Das, B.; Gogoi, P. Org. Biomol. Chem. 2018, 16, 3138. |
| [49] | Sharma, A.; Gogoi, P. Org. Biomol. Chem. 2019, 17, 333. |
| [50] | Zhou, M.; Ni, C.; Zeng, Y.; Hu, J. J. Am. Chem. Soc. 2018, 140, 6801. |
| [51] | Lei, M.; Miao, H.; Zhu, C. J.; Lu, X. Q.; Shen, J.; Qin, Y. R.; Zhang, H. Y.; Sha, S. J.; Zhu, Y. Q. Tetrahedron Lett. 2019, 60, 1389. |
| [52] | Huang, W. B.; Qiu, L. Q.; Ren, F. Y.; He, L. N. Chem. Commun. 2021, 57, 9578. |
| [53] | Yoshida, H.; Fukushima, H.; Ohshita, J.; Kunai, A. J. Am. Chem. Soc. 2006, 128, 11040. |
| [54] | Yoshida, H.; Morishita, T.; Ohshita, J. Org. Lett. 2008, 10, 3845. |
| [55] | Yoshida, H.; Morishita, T.; Fukushima, H.; Ohshita, J.; Kunai, A. Org. Lett. 2007, 9, 3367. |
| [56] | Morishita, T.; Fukushima, H.; Yoshida, H.; Ohshita, J.; Kunai. A. Asian J. Org. Chem. 2008, 73, 5452. |
| [57] | Giumanini, A. G. J. Org. Chem. 1972, 37, 513. |
| [58] | Yan, Q.; Fan, R.; Liu, B. B.; Su, S. S.; Wang, B.; Yao, T. L.; Tan, J. J. Chin. J. Org. Chem. 2021, 41, 455. (in Chinese) |
| [58] | (闫强, 范荣, 刘斌斌, 苏帅松, 王勃, 姚团利, 谭嘉靖, 有机化学, 2021, 41, 455.) |
| [59] | Masilamani, J.; Sivakolundu, B. J. Org. Chem. 2010, 5, 153. |
| [60] | Stephens, D.; Zhang, Y.; Cormier, M.; Chavez, G.; Arman, H.; Larionov, O. V. Chem. Commun. 2013, 49, 6558. |
| [61] | Tang, C. Y.; Wang, G.; Yang, X. Y.; Wu, X. Y.; Sha, F. Tetrahedron Lett. 2014, 55, 6447. |
| [62] | Kaldas, S. J.; Kran, E.; Mu?ck, C.; Yudin, A. K.; Studer, A. Chem.- Eur. J. 2020, 26, 1501. |
| [63] | Roy, T.; Baviskar, D. R.; Biju, A. T. J. Org. Chem. 2015, 80, 11131. |
| [64] | Roy, T.; Bhojgude, S. S.; Kaicharla, T.; Thangaraj, M.; Garai, B.; Biju, A. T. Org. Chem. Front. 2016, 3, 71. |
| [65] | Roy, T.; Thangaraj, M.; Gonnade, R. G.; Biju, A. T. Chem. Commun. 2016, 52, 9044. |
| [66] | Bhunia, A.; Roy, T.; Pachfule, P.; Rajamohanan, P. R.; Biju, A. T. Angew. Chem., Int. Ed. 2013, 52, 10040. |
| [67] | Bhunia, A.; Porwal, D.; Gonnade, R. G.; Biju, A. T. Org. Lett. 2013, 15, 4620. |
| [68] | Liu, P.; Lei, M.; Hu, L. Tetrahedron 2013, 69, 10405. |
| [69] | Zhou, Y.; Chi, Y.; Zhao, F.; Zhang, W. X.; Xi, Z. Chem.-Eur. J. 2014, 20, 2463. |
| [70] | Bhojgude, S. S.; Baviskar, D. R.; Gonnade, R. G.; Biju, A. T. Org. Lett. 2015, 17, 6270. |
| [71] | Liu, K.; Gu, C. Z.; Dai, B.; He, L. RSC Adv. 2016, 6, 33606. |
| [72] | Bhojgude, S. S.; Roy, T.; Gonnade, R. G.; Biju, A. T. Org. Lett. 2016, 18, 5424. |
| [73] | Okuma, K.; Kinoshita, H.; Nagahora, N.; Shioji, K. Eur. J. Org. Chem. 2016, 2264. |
| [74] | Shu, W. M.; Ma, J. R.; Zheng, K. L.; Wu, A. X. Org. Lett. 2016, 18, 196. |
| [75] | Suh, S. E.; Chenoweth, D. M. Org. Lett. 2016, 18, 4080. |
| [76] | Jeganmohan, M.; Cheng, C. H. Chem. Commun. 2006, 2454. |
| [77] | Tan, J.; Liu, B.; Su, S. Org. Chem. Front. 2018, 5, 3093. |
| [78] | Liu, K.; Liu, L. L.; Gu, C. Z.; Dai, B.; He, L. RSC Adv. 2016, 6, 33606. |
| [79] | Xu, J. K.; Li, S. J.; Wang, H. Y.; Xu, W. C.; Tian, S. K. Chem. Commun. 2017, 53, 1708. |
| [80] | Guranova, N. I.; Voskressensky, L. G. Mendeleev Commun. 2017, 27, 506. |
| [81] | Phatake, R. S.; Mullapudi, V.; Wakchaure, V. C.; Ramana, C. V. Org. Lett. 2017, 19, 372. |
| [82] | Gui, Y.; Tian, S. K. Org. Lett. 2017, 19, 1554. |
| [83] | Min, G.; Seo, J.; Ko, H. M. J. Org. Chem. 2018, 83, 8417. |
| [84] | Seo, J.; Kim, D.; Ko, H. M. Adv. Synth. Catal. 2020, 362, 2739. |
| [85] | Li, S. J.; Wang, Y.; Xu, J. K.; Xie, D.; Tian, S. K.; Yu, Z. X. Org. Lett. 2018, 20, 4545. |
| [86] | Huang, X.; Zhao, W.; Chen, D. L.; Zhan, Y.; Zeng, T.; Jin, H.; Peng, B. Chem. Commun. 2019, 55, 2070. |
| [87] | Li, S. J.; Han, L.; Tian, S. K. Chem. Commun. 2019, 55, 11255. |
| [88] | Dhokale, R. A.; Mhaske, S. B. Org. Lett. 2016, 18, 3010. |
| [89] | Kwon, J.; Kim, B. M. Org. Lett. 2019, 21, 428. |
| [90] | Yoshida, H.; Fukushima, H.; Ohshita, J.; Kunai, A. Angew. Chem., Int. Ed. 2004, 43, 3935. |
| [91] | Yoshida, H.; Ohshita, J.; Kunai, A. Tetrahedron Lett. 2004, 45, 8659. |
| [92] | Yoshida, H.; Kunai, S. Tetrahedron 2007, 63, 4793. |
| [93] | Xie, C. S.; Zhang, Y. H.; Xu, P. X. Synlett 2008, 3115. |
| [94] | Huang, X.; Zhang, T. Tetrahedron Lett. 2009, 50, 208. |
| [95] | Sha, F.; Huang, X. Angew. Chem., Int. Ed. 2009, 48, 3458. |
| [96] | Sha, F.; Wu, L.; Huang, X. J. Org. Chem. 2012, 77, 3754. |
| [97] | Shen, H.; Wu, X. Y. Eur. J. Org. Chem. 2013, 13, 2537. |
| [98] | Gilmore, C. D.; Stoltz, B. M. Angew. Chem., Int. Ed. 2011, 50, 4488. |
| [99] | Yoshida, H.; Asatsu, Y.; Mimura, Y.; Ito, Y.; Ohshita, J.; Takaki, K. Angew. Chem., Int. Ed. 2011, 50, 9676. |
| [100] | Kaicharla, T.; Thangaraj, M.; Biju, A. T. Org. Lett. 2014, 16, 1728. |
| [101] | Fang, Y.; Wang, S. Y.; Ji, S. J. Tetrahedron 2015, 71, 2768. |
| [102] | Serafini, M.; Griglio, A.; Viarengo, S.; Aprile, S.; Pirali, T. Org. Biomol. Chem. 2017, 15, 6604. |
| [103] | Li, R.; Wu, C. R.; Shi, F. J. Org. Chem. 2014, 79, 1344. |
| [104] | Zhang, Y.; Xiong, W.; Cen, J.; Wu, Y.; Qi, C.; Wu, W.; Jiang, H. Chem. Commun. 2019, 55, 12304. |
| [105] | Shu, W. M.; Zheng, K. L.; Ma, J. R.; Wu, A. X. Org. Lett. 2016, 18, 3762. |
| [106] | Huang, X.; Xue, J. J. Org. Chem. 2007, 72, 3965. |
| [107] | Shu, W. M.; Liu, S.; He, J. X.; Wang, S.; Wu, A. X. J. Org. Chem. 2018, 83, 9156. |
| [108] | Zeng, Y.; Li, G.; Hu, J. Angew. Chem., Int. Ed. 2015, 54, 10773. |
| [109] | Okugawa, Y.; Hayashi, Y.; Kawauchi, S.; Hirano, K.; Miura, M. Org. Lett. 2018, 20, 3670. |
| [110] | Xiong, W.; Qi, C.; Cheng, R.; Zhang, H.; Wang, L.; Yan, D.; Jiang, H. Chem. Commun. 2018, 54, 5835. |
| [111] | Bhattacharjee, S.; Guin, A.; Gaykar, R. N.; Biju, A. T. Org. Lett. 2019, 21, 4383. |
| [112] | Jiang, H.; Zhang, Y.; Xiong, W.; Cen, J.; Wang, L.; Cheng, R.; Qi, C.; Wu, W. Org. Lett. 2019, 21, 345. |
| [113] | Fujimoto, H.; Kusano, M.; Kodama, T.; Tobisu, M. Org. Lett. 2020, 22, 229. |
/
| 〈 |
|
〉 |